Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Qualitative Immunoblot/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Qualitative Immunoblot/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Autoimmune encephalopathy and autoimmune dementia are rare but important reversible causes of cognitive impairment and decline. Recognition of autoimmune causes of neurologic symptoms and detection of antineural antibodies may help to establish a diagnosis, support treatment decisions, aid with prognostication, serve as a prerequisite for enrollment in clinical trials, and guide the search for an associated malignancy.
Disease Overview
Autoimmune encephalopathy and autoimmune dementia may develop in the context of paraneoplastic neurologic syndromes or postinfectious syndromes but are most often idiopathic. A diagnosis of possible autoimmune encephalitis should be considered if there is a subacute onset or rapid progression (in <3 months) of short-term memory loss, altered level of consciousness, lethargy, personality change, or psychiatric symptoms as well as either new focal central nervous system (CNS) findings, seizures without another explanation, cerebrospinal fluid (CSF) pleocytosis, or magnetic resonance imaging (MRI) features suggestive of encephalitis, with reasonable exclusion of alternative causes. Although antibody testing is not required for the diagnosis of possible autoimmune encephalitis, it plays an important role in increasing the level of certainty of the diagnosis to probable or definite. The Antibody Prevalence in Epilepsy and Encephalopathy (APE2) score can be used when considering autoimmune causes of encephalopathy and dementia to determine the likelihood that antineural antibodies will be present.
For more information about laboratory testing for autoimmune neurologic diseases, refer to the ARUP Consult Autoimmune Neurologic Diseases - Antineural Antibody Testing topic.
Test Description
ARUP’s serum and CSF Autoimmune Encephalopathy/Dementia Panels can be used for the evaluation of patients with new, acute- to subacute-onset encephalopathy, dementia, or cognitive impairment. Testing for the presence of antineural antibodies in both serum and CSF may improve diagnostic yield.
These phenotype-targeted panels test for the presence of antibodies associated with autoimmune encephalopathy and autoimmune dementia. Clinical phenotypes for specific antineural antibody-associated syndromes often overlap, and phenotype-specific panels allow for rapid identification of associated antibodies, which may have implications for treatment, prognosis, cancer screening, and clinical trial enrollment. Other panels may be more appropriate, depending on the patient’s clinical phenotype:
| ARUP Panel |
|
|
|---|---|---|
| Serum | CSF | |
|
Autoimmune Epilepsy Panel |
||
|
Autoimmune Movement Disorder Panel |
||
|
Autoimmune Pediatric CNS Disorders Panel |
||
Regardless of the panel chosen, order only one panel for serum and/or one panel for CSF; many antineural antibodies are redundant between these panels, and choosing based on the predominant phenotype will provide the most meaningful results. To compare these panels and the antibodies included, refer to ARUP Autoimmune Neurology Panel Components.
Testing for individual antibodies is also available separately.
Antibodies Tested and Methodology
| Autoantibody Markers | Methodology |
|
|
|---|---|---|---|
| Serum | CSF | ||
|
AMPAR Ab, IgG |
CBA-IFA, reflex titer |
||
|
Amphiphysin Ab, IgG |
IB |
||
|
ANNA-1 (Hu) |
IFA, reflex IB, reflex titer |
||
|
ANNA-2 (Ri) |
IFA, reflex IB, reflex titer |
||
|
CASPR2 Ab, IgG |
CBA-IFA, reflex titer |
||
|
CV2 (CRMP-5) Ab, IgG |
CBA-IFA, reflex titer |
||
|
DPPX Ab, IgG |
CBA-IFA, reflex titer |
||
|
GABA-BR Ab, IgG |
CBA-IFA, reflex titer |
||
|
GAD65 Ab |
ELISA |
||
|
IgLON5 Ab, IgG |
CBA-IFA, reflex titer |
||
|
LGI1 Ab, IgG |
CBA-IFA, reflex titer |
||
|
mGluR1 Ab, IgG |
CBA-IFA, reflex titer |
||
|
NMDAR Ab, IgG |
CBA-IFA, reflex titer |
||
|
PCCA-1 (Yo) |
IFA, reflex IB, reflex titer |
||
|
PCCA-Tr/DNER |
IFA, reflex IB, reflex titer |
||
|
SOX1 (AGNA) Ab, IgG |
IB |
||
|
Ab, antibody; AGNA, antiglial nuclear antibody; AMPAR, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ANNA, antineuronal nuclear antibody type 1; ANNA-2, antineuronal nuclear antibody type 2; CASPR2, contactin-associated protein 2; CBA, cell-binding assay/cell-based assay; CRMP-5, collapsin response-mediator protein 5; DNER, Delta/notch-like epidermal growth factor-related receptor; DPPX, dipeptidyl-aminopeptidase-like protein 6; ELISA, enzyme-linked immunosorbent assay; GABA-BR, gamma-aminobutyric acid receptor, type B; GAD65, glutamic acid decarboxylase 65-kd isoform; IB, immunoblot; IFA, indirect immunofluorescence assay; IgG, immunoglobulin G; IgLON5, IgLON family member 5; LGl1, leucine-rich, glioma-inactivated protein 1; mGluR1, metabotropic glutamate receptor 1; NMDAR, N-methyl-D-aspartate receptor; PCCA-1, Purkinje cell cytoplasmic antibody type 1; PCCA-Tr, Purkinje cell cytoplasmic antibody type Tr; SOX1, SRY-box transcription factor 1 |
|||
Reflex Patterns
Autoimmune Encephalopathy/Dementia Panel, Serum (3006201) and CSF (3006202): Reflex Patterns
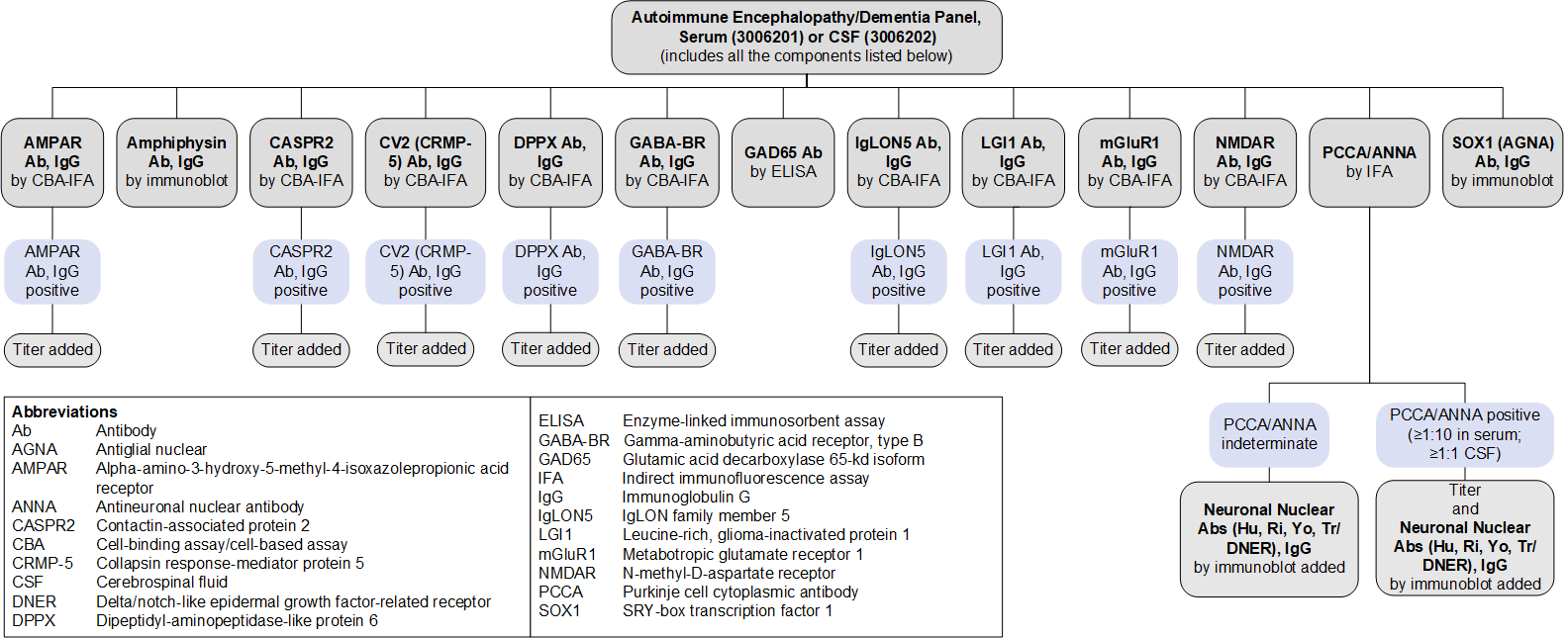
Limitations
These panels do not include every antibody that has been associated with autoimmune dementia or encephalopathy:
- ANNA-3 and PCCA-2 are not included because they are extremely rare (present in approximately 0.0001% of specimens submitted for evaluation using a paraneoplastic antibody panel), and commercial assays to confirm the specificity of these antibodies are not currently available.
- Glial fibrillary acidic protein (GFAP), neuronal intermediate filament (NIF) and its associated reflexes (NIF heavy and light chain, alpha internexin), neurochondrin, and septin 7 are not included because they have been only recently identified and their prevalence is currently not well established.
- GFAP has been reported in 0.17% of samples screened, often co-occurring with other antineural antibodies.
- NIF has been reported in 0.014% of samples screened; NIF heavy and light chain and alpha internexin were reflexed in samples which were positive for NIF to further identify the associated antibody.
- Neurochondrin has been reported in 0.002% of samples tested.
- Septin 7 has been reported in 0.002% of samples screened.
- As testing for newly described antibodies becomes available and their clinical relevance is established, these panels will evolve to reflect these discoveries.
Test Interpretation
Results
Results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
|
Positive for ≥1 autoantibodies |
Autoantibody(ies) detected Supports a clinical diagnosis of an autoimmune encephalopathy or autoimmune dementia Consider a focused search for malignancy based on antibody-tumor associations |
|
Negative |
No autoantibodies detected A diagnosis of an autoimmune encephalopathy or autoimmune dementia is not excluded |
References
-
34341093
Bastiaansen AEM, van Steenhoven RW, de Bruijn MAAM, et al. Autoimmune encephalitis resembling dementia syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1039.
-
27112681
Flanagan EP, Drubach DA, Boeve BF. Autoimmune dementia and encephalopathy. Handb Clin Neurology. 2016;133:247-267.
-
26906964
Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
-
30196836
Dubey D, Kothapalli N, McKeon A, et al. Predictors of neural-specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol. 2018;323:62-72.
-
24833664
Horta ES, Lennon VA, Lachance DH, et al. Neural autoantibody clusters aid diagnosis of cancer. Clin Cancer Res. 2014;20(14):3862-3869.
-
29293273
Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166-177.
-
30282771
Basal E, Zalewski N, Kryzer TJ, et al. Paraneoplastic neuronal intermediate filament autoimmunity. Neurology. 2018;91(18):e1677-e1689.
-
31511329
Shelly S, Kryzer TJ, Komorowski L, et al. Neurochondrin neurological autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e612.
-
36053822
Hinson SR, Honorat JA, Grund EM, et al. Septin‐5 and ‐7‐IgGs: neurologic, serologic, and pathophysiologic characteristics. Ann Neurol. 2022;92(6):1090-1101.



 Feedback
Feedback
ARUP Autoimmune Neurology Panel Components