Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Qualitative Immunoblot/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Qualitative Immunoblot/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Autoimmune epilepsy is characterized by acute to subacute onset of epilepsy that is often refractory to standard treatment with antiseizure drugs but responds to immunotherapy. Recognition of autoimmune causes of neurologic symptoms and detection of antineural antibodies may help to establish a diagnosis, support treatment decisions, serve as a prerequisite for enrollment in clinical trials, and guide the search for an associated malignancy.
Disease Overview
Autoimmune epilepsy accounts for 15-20% of epilepsies previously considered to be cryptogenic. In patients with new-onset seizure activity, the Antibody Prevalence in Epilepsy and Encephalopathy (APE2) score can be used to predict the likelihood of the presence of an antineural antibody and should therefore be considered before ordering antineural antibody testing. Factors associated with autoimmune epilepsy include autonomic dysfunction, brain magnetic resonance imaging (MRI) findings suggestive of encephalitis, elevated cerebrospinal fluid (CSF) protein or pleocytosis, faciobrachial dystonic seizures, history of autoimmunity, history of malignancy, neuropsychiatric changes, orofacial dyskinesias, seizures refractory to antiseizure drugs, and viral prodrome. It is important to note that autoimmune epilepsy may exist in the absence of detectable, known antineural antibodies, and empiric immunotherapy trials may be considered in the appropriate clinical context.
For more information about laboratory testing for autoimmune neurologic diseases, refer to the ARUP Consult Autoimmune Neurologic Disease - Antineural Antibody Testing topic.
Test Description
These serum and CSF antineural antibody panel tests can be used for the evaluation of patients with a neurologic phenotype consisting predominantly of new, acute to subacute onset of epilepsy that is refractory to more than two antiseizure medications. Testing for the presence of antineural antibodies in both serum and CSF is recommended to improve diagnostic yield.
These phenotype-targeted panels test for the presence of antibodies associated with epilepsy. Clinical phenotypes for specific antineural antibody-associated syndromes often overlap, and phenotype-specific panels allow for rapid identification of associated antibodies, which may have implications for treatment, prognosis, and cancer screening. Other panels may be more appropriate, depending on the patient’s clinical phenotype:
| ARUP Panel | Test Code | |
|---|---|---|
| Serum | CSF | |
| Autoimmune Movement Disorder Panel | 3018964 | 3018966 |
| Autoimmune Encephalopathy/Dementia Panel | 3006201 | 3006202 |
| Autoimmune Pediatric CNS Disorders Panel | 3006210 | 3006211 |
Regardless of the panel chosen, order only one panel for serum and/or one panel for CSF; many antineural antibodies are redundant between these panels, and choosing based on the predominant phenotype will provide the most meaningful results. To compare these panels and the antibodies included, refer to the ARUP Antineural Antibody Testing for Autoimmune Neurologic Disease page.
Testing for individual antibodies is also available separately.
Antibodies Tested and Methodology
| Autoantibody Markers | Methodology | Individual Autoantibody or Focused Panel Test Code | |
|---|---|---|---|
| Serum | CSF | ||
| AMPAR Ab, IgG | CBA-IFA, reflex titer | 3001260 | 3001257 |
| Amphiphysin Ab, IgG | IB | 2008893 | 3004510 |
| ANNA-1 (Hu) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| ANNA-2 (Ri) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| CASPR2 Ab, IgG | CBA-IFA, reflex titer | 2009452 | 3001986 |
| CV2 (CRMP-5) Ab, IgG | CBA-IFA, reflex titer | 3016999 | 3017001 |
| DPPX Ab, IgG | CBA-IFA, reflex titer | 3004359 | 3004512 |
| GABA-AR Ab, IgG | CBA-IFA, reflex titer | 3006008 | 3006003 |
| GABA-BR Ab, IgG | CBA-IFA, reflex titer | 3001270 | 3001267 |
| GAD65 Ab | ELISA | 2001771 | 3002788 |
| LGI1 Ab, IgG | CBA-IFA, reflex titer | 2009456 | 3001992 |
| Ma2/Ta Ab, IgG | IB | 3017441 | 3017440 |
| mGluR1 Ab, IgG | CBA-IFA, reflex titer | 3006044 | 3006039 |
| NMDAR Ab, IgG | CBA-IFA, reflex titer | 2004221 | 2005164 |
| PCCA-1 (Yo) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| PCCA-Tr/DNER | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| SOX1 (AGNA) Ab, IgG | IB | 3002885 | 3002886 |
| Ab, antibody; AGNA, antiglial nuclear antibody; AMPAR, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ANNA-1, antineuronal nuclear antibody type 1; ANNA-2, antineuronal nuclear antibody type 2; CASPR2, contactin-associated protein 2; CBA, cell-binding assay/cell-based assay; CRMP-5, collapsin response-mediator protein 5; DNER, Delta/notch-like epidermal growth factor-related receptor; DPPX, dipeptidyl-aminopeptidase-like protein 6; ELISA, enzyme-linked immunosorbent assay; GABA-AR, gamma-aminobutyric acid receptor, type A; GABA-BR, gamma-aminobutyric acid receptor, type B; GAD65, glutamic acid decarboxylase 65-kd isoform; IB, immunoblot; IFA, indirect immunofluorescence assay; LGl1, leucine-rich, glioma-inactivated protein 1; mGluR1, metabotropic glutamate receptor 1; NMDAR, N-methyl-D-aspartate receptor; PCCA, Purkinje cell cytoplasmic antibody; SOX1, SRY-box transcription factor 1 | |||
Reflex Patterns
Autoimmune Epilepsy Panel, Serum (3006204) and Autoimmune Epilepsy Panel, CSF (3006205): Reflex Patterns
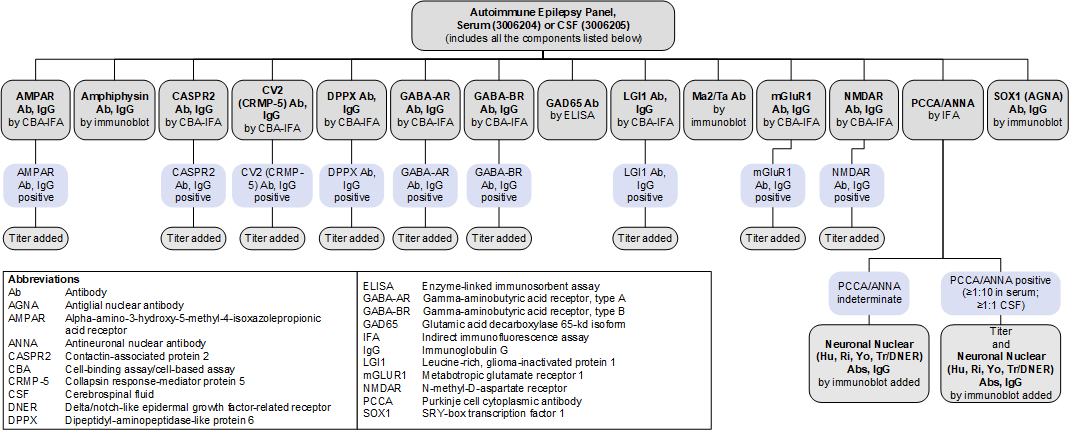
Limitations
This panel does not include every antibody that has been associated with autoimmune epilepsy:
- ANNA-3 and PCCA-2 are not included in this panel because they are extremely rare (present in approximately 0.0001% of specimens submitted for evaluation using a paraneoplastic antibody panel), and commercial assays to confirm the specificity of these antibodies are not currently available.
- Glial fibrillary acidic protein (GFAP) and neurochondrin are not included because they have been only recently identified and their prevalence is currently not well established.
- As testing for newly described antibodies becomes available and their clinical relevance is established, these panels will evolve to reflect these discoveries.
Test Interpretation
Results
Results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected Supports a clinical diagnosis of autoimmune epilepsy Consider a focused search for malignancy based on antibody-tumor associations |
| Negative | No autoantibodies detected A diagnosis of autoimmune epilepsy is not excluded |
References
-
33029957
Jang Y, Kim DW, Yang KI, et al. Clinical approach to autoimmune epilepsy. J Clin Neurol. 2020;16(4):519-529.
-
28555833
Dubey D, Singh J, Britton JW, et al. Predictive models in the diagnosis and treatment of autoimmune epilepsy. Epilepsia. 2017;58(7):1181-1189.
-
30196836
Dubey D, Kothapalli N, McKeon A, et al. Predictors of neural-specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol. 2018;323:62-72.
-
27037228
Lee WJ, Lee ST, Byun JI, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology. 2016;86(18):1683-1691.
-
27112681
Flanagan EP, Drubach DA, Boeve BF. Autoimmune dementia and encephalopathy. Handb Clin Neurology. 2016;133:247-267.
-
33221892
Budhram A, Dubey D, Sechi E, et al. Neural antibody testing in patients with suspected autoimmune encephalitis. Clin Chem. 2020;66(12):1496-1509.
-
24833664
Horta ES, Lennon VA, Lachance DH, et al. Neural autoantibody clusters aid diagnosis of cancer. Clin Cancer Res. 2014;20(14):3862-3869.
-
29293273
Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166-177.
-
31511329
Shelly S, Kryzer TJ, Komorowski L, et al. Neurochondrin neurological autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e612.


 Feedback
Feedback