Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Qualitative Immunoblot/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Semi-Quantitative Cell-Based Indirect Fluorescent Antibody/Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Qualitative Immunoblot/Semi-Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
Autoimmune myelopathy should be considered in patients who present with subacute onset and rapid progression of symptoms associated with spinal cord dysfunction, such as weakness, sensory loss, gait difficulties, or bowel and bladder dysfunction. The differential in these conditions is broad, and in conjunction with clinical history, neurologic exam, imaging, and other laboratory studies, evaluation of these disorders with a phenotype-specific autoimmune myelopathy antibody panel may help to establish a diagnosis, guide treatment plans and prognostication, and assist in a targeted search for an associated malignancy.
Disease Overview
Myelopathy may be due to many etiologies, including infections, cord compression, vascular anomalies, metabolic derangements, malignancy, multiple sclerosis, granulomatous disease, and autoimmune causes. Although imaging findings and clinical exam and history may provide clues to the etiology, laboratory testing including a myelopathy phenotype-specific antineural antibody panel is an important component of a diagnostic evaluation. Treatment of autoimmune myelopathy is distinct from that of myelopathy due to other causes, and prompt evaluation and initiation of treatment can reduce morbidity.
For more information about laboratory testing for autoimmune neurologic diseases, refer to the ARUP Consult Autoimmune Neurologic Diseases - Antineural Antibody Testing topic.
Test Description
These serum and CSF antineural antibody panel tests can be used for the evaluation of patients with rapid onset of neurologic symptoms localizing to the spinal cord. Testing for the presence of antineural antibodies in both serum and CSF may improve diagnostic yield.
These phenotype-targeted panels test for the presence of antibodies associated with myelopathy. Clinical phenotypes for specific antineural antibody-associated syndromes often overlap, and phenotype-specific panels allow for rapid identification of associated antibodies, which may have implications for treatment, prognosis, and cancer screening.
In patients <18 years of age, consider ARUP’s Autoimmune Pediatric CNS Disorders Panel in serum (3006210) or CSF (3006211).
Testing for individual antibodies is also available separately.
Antibodies Tested and Methodology
| Autoantibody Markers | Methodology | Individual Autoantibody Test Code | |
|---|---|---|---|
| Serum | CSF | ||
| Amphiphysin Ab, IgG | IB | 2008893 | 3004510 |
| ANNA-1 (Hu) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| ANNA-2 (Ri) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| AQP4 Ab, IgG | CBA-IFA, reflex titer | 2013320 | 2011699 |
| CV2 (CRMP-5) Ab, IgG | CBA-IFA, reflex titer | 3016999 | 3017001 |
| DPPX Ab, IgG | CBA-IFA, reflex titer | 3004359 | 3004512 |
| GABA-BR Ab, IgG | CBA-IFA, reflex titer | 3001270 | 3001267 |
| GAD65 Ab | ELISA | 2001771 | 3002788 |
| mGluR1 Ab, IgG | CBA-IFA, reflex titer | 3006044 | 3006039 |
| MOG Ab, IgG | CBA-IFA, reflex titer | 3001277 | — |
| PCCA-1 (Yo) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| PCCA-Tr/DNER | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| SOX1 (AGNA) Ab, IgG | IB | 3002885 | 3002886 |
| Ab, antibody; AGNA, antiglial nuclear antibody; ANNA-1, antineuronal nuclear antibody type 1; ANNA-2, antineuronal nuclear antibody type 2; AQP4, aquaporin 4; CBA, cell-binding assay/cell-based assay; CRMP-5, collapsin response-mediator protein 5; DNER, Delta/notch-like epidermal growth factor-related receptor; DPPX, dipeptidyl-aminopeptidase-like protein 6; ELISA, enzyme-linked immunosorbent assay; GABA-BR, gamma-aminobutyric acid receptor, type B; GAD65, glutamic acid decarboxylase 65-kd isoform; IB, immunoblot; IFA, indirect immunofluorescence assay; mGluR1, metabotropic glutamate receptor 1; MOG, myelin oligodendrocyte glycoprotein; PCCA, Purkinje cell cytoplasmic antibody; SOX1, SRY-box transcription factor 1 | |||
Reflex Patterns
Autoimmune Myelopathy Panel, Serum (3006208) and CSF (3006209): Reflex Patterns
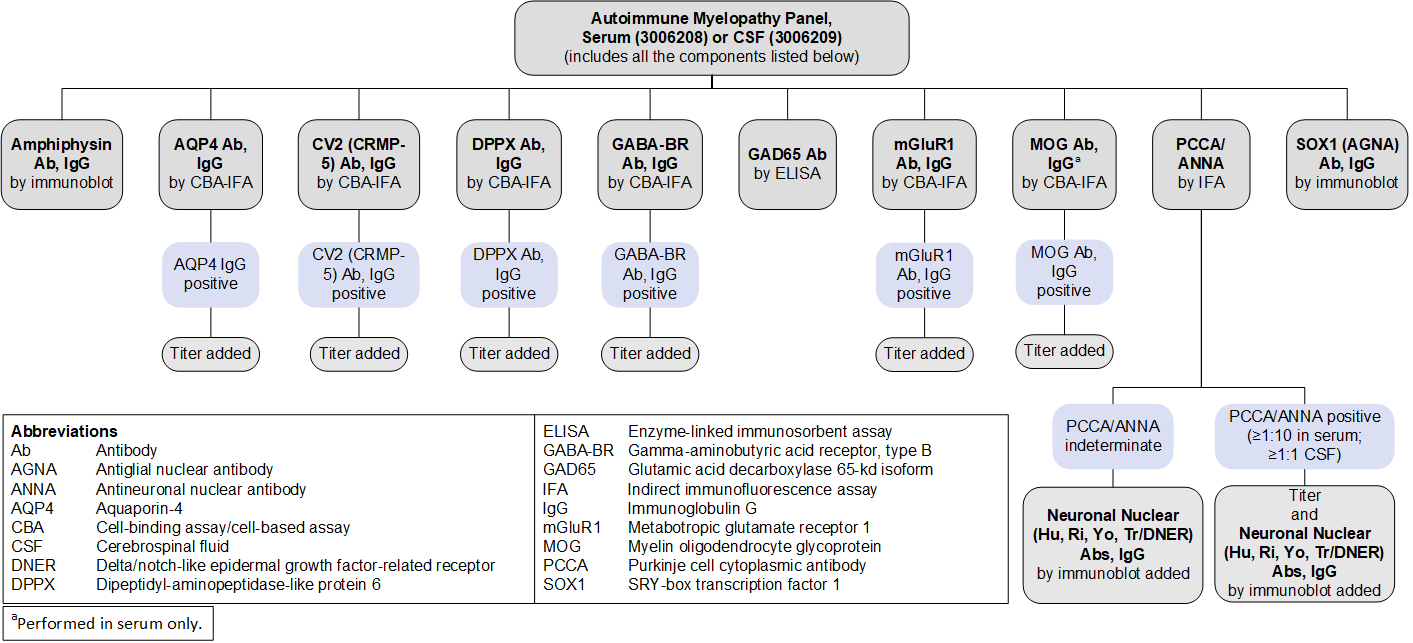
Limitations
These panels do not include every antibody that has been associated with autoimmune myelopathy:
- ANNA-3 and PCCA-2 are not included in this panel because they are extremely rare (present in approximately 0.0001% of specimens submitted for evaluation using a paraneoplastic antibody panel), and commercial assays to confirm the specificity of these antibodies are not currently available.
- Adaptor protein 3 subunit B2 (AP3B2), glial fibrillary acidic protein (GFAP), GTPase regulator associated with focal adhesion kinase 1 (GRAF1), neuronal intermediate filament (NIF) and its associated reflexes (NIF heavy and light chain, alpha internexin), neurochondrin, and septin 7 antibodies are not included because they have been only recently identified and their prevalence is currently not well established.
- AP3B2 has been reported in <0.002% of samples screened.
- GFAP has been reported in 0.17% of samples screened, often co-occurring with other antineural antibodies.
- NIF has been reported in 0.014% of samples screened; NIF heavy and light chain and alpha internexin were reflexed in samples that were positive for NIF to further identify the associated antibody.
- Neurochondrin has been reported in 0.002% of samples tested.
- Septin 7 has been reported in 0.002% of samples screened.
- As testing for newly described antibodies becomes available and their clinical relevance is established, these panels will evolve to reflect these discoveries.
Test Interpretation
Results
Results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected Supports a clinical diagnosis of autoimmune myelopathy Consider a focused search for malignancy based on antibody-tumor associations |
| Negative | No autoantibodies detected A diagnosis of autoimmune myelopathy is not excluded |
References
-
27112686
Flanagan EP. Autoimmune myelopathies. Handb Clin Neurol. 2016;133:327-351.
-
35968301
Passeri M, Matthews E, Kammeyer R, et al. Update in autoimmune and paraneoplastic myelopathies: newly described antigen targets and antibody testing. Front Neurol. 2022;13:972143.
-
27112681
Flanagan EP, Drubach DA, Boeve BF. Autoimmune dementia and encephalopathy. Handb Clin Neurology. 2016;133:247-267.
-
24833664
Horta ES, Lennon VA, Lachance DH, et al. Neural autoantibody clusters aid diagnosis of cancer. Clin Cancer Res. 2014;20(14):3862-3869.
-
31371564
Honorat JA, Lopez-Chiriboga AS, Kryzer TJ, et al. Autoimmune gait disturbance accompanying adaptor protein-3B2-IgG. Neurology. 2019;93(10):e954-e963.
-
29293273
Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166-177.
-
30282771
Basal E, Zalewski N, Kryzer TJ, et al. Paraneoplastic neuronal intermediate filament autoimmunity. Neurology. 2018;91(18):e1677-e1689.
-
31511329
Shelly S, Kryzer TJ, Komorowski L, et al. Neurochondrin neurological autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e612.
-
36053822
Hinson SR, Honorat JA, Grund EM, et al. Septin‐5 and ‐7‐IgGs: neurologic, serologic, and pathophysiologic characteristics. Ann Neurol. 2022;92(6):1090-1101.


 Feedback
Feedback