Chromogenic Assay
Chromogenic Assay
- Use to measure apixaban or rivaroxaban concentration.
- To assess steady-state trough levels, draw should occur at the end of a dosing interval or immediately prior to a DOAC dose.
- To assess steady-state peak levels, draw should occur 3-4 hours after an apixaban dose or 2-4 hours after a rivaroxaban dose.
A subset of direct oral anticoagulants (DOACs) directly inhibit coagulation factor Xa. These direct Xa inhibitors do not require routine laboratory monitoring. However, there are some clinical scenarios in which efficacy or safety may be a concern and a measurement of direct Xa inhibitor activity may be useful. Drug-specific anti-Xa activity assays calibrated for a single direct Xa inhibitor may be used to make quantitative measurements of drug level. There is currently no definitive clinical trial evidence for adjusting apixaban, edoxaban, or rivaroxaban dose based on an anti-Xa activity result.
Testing Strategy
While routine monitoring is not required, measurement of direct Xa inhibitor (e.g., apixaban, rivaroxaban) levels may be helpful in contexts that would affect their safe and effective use, such as impaired absorption, drug interaction, treatment failure, or renal impairment. The following algorithm provides a testing strategy for measuring these direct Xa inhibitors. For additional information on reported trough and peak concentration ranges, see the On-Therapy Range Information table.
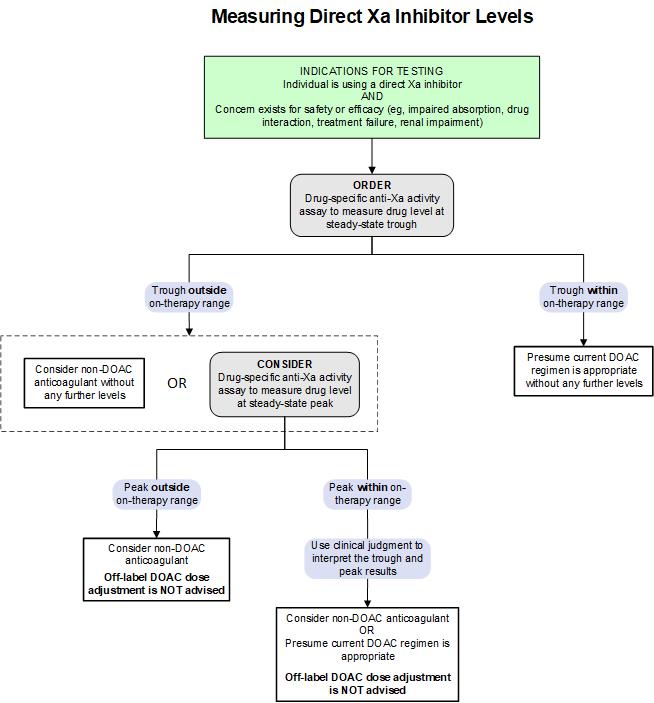
Test Interpretation
Clinical and/or Analytic Sensitivity
Lower limit of assay detection by drug:
- Apixaban: 23 ng/mL
- Rivaroxaban: 25 ng/mL
Results
- The On-Therapy Range Information table comes from published data (including pharmacokinetic studies and phase-three clinical trials , , ) and applies to adults ≥18 years of age. Trough and peak concentration ranges represent the median (e.g., 5th-95th percentile) for the studied population; they are NOT meant to be interpreted as therapeutic concentration ranges.
- ARUP interpretive comments will include on-therapy ranges from published data regarding treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE).
| Drug | Dose | Indication | Observed Trough (5th-95th percentile except as otherwise indicated) | Observed Peak (5th-95th percentile except as otherwise indicated) |
|---|---|---|---|---|
| Apixaban | 10 mg twice daily | Treatment of DVT and PE (First 7 days of therapy) | 41-335 ng/mL | 111-572 ng/mL |
| 5 mg twice daily | Treatment of DVT and PE | 22-177 ng/mL | 59-302 ng/mL | |
| 5 mg twice daily | Stroke prevention in nonvalvular atrial fibrillation | 41-230 ng/mL | 91-321 ng/mL | |
| 2.5 mg twice daily | Stroke prevention in nonvalvular atrial fibrillation (reduced-dose population) | 34-162 ng/mL "> | 69-221 ng/mL "> | |
| 2.5 mg twice daily | Prevention of VTE following elective hip or knee replacement surgery | 23-109 ng/mL "> | 41-146 ng/mL | |
| 2.5 mg twice daily | Prevention of recurrent VTE | 11-90 ng/mL | 30-153 ng/mL | |
| Rivaroxaban | 20 mg daily | Treatment of DVT and PE | 6-87 ng/mL | 189-419 ng/mL |
| 20 mg daily | Stroke prevention in nonvalvular atrial fibrillation | 12-137 ng/mL | 184-343 ng/mL | |
| 15 mg daily | Stroke prevention in nonvalvular atrial fibrillation (reduced-dose population) | 18-136 ng/mL | 178-313 ng/mL | |
| 10 mg daily | Prevention of VTE following elective hip or knee replacement surgery | 1-38 ng/mL | 91-196 ng/mL | |
| 10 mg daily | Prevention of recurrent VTE | 4-51 ng/mL (10th-90th percentile) | 7-273 ng/mL (10th-90th percentile) | |
aFor more details, see References and Additional Resources. VTE, venous thromboembolism Sources: Douxfils, 2021 ; European Medicines Agency, 2021 ; Mueck, 2014 | ||||
Limitations
- Anti-Xa activity assays can only be used to accurately measure the specific drug for which they are calibrated (e.g., apixaban can only be accurately measured in an anti-Xa assay that includes an apixaban calibrator).
- Anti-Xa activity results are not reliable when more than one drug with anti-Xa activity is present in a plasma sample. Any drug with activity against factor Xa (e.g., unfractionated heparin, low molecular weight heparin, fondaparinux, apixaban, edoxaban, rivaroxaban) will show effect in any of the anti-Xa activity assays. That is, if more than one drug is present (e.g., unfractionated heparin and rivaroxaban), the effects of both drugs will be detected in the anti-Xa activity assay, regardless of the drug for which the assay is calibrated.
- Grossly hemolyzed specimens are not suitable for testing due to potential coagulation factor activation and chromogenic assay interference.
- Grossly icteric samples are not suitable for testing as bilirubin may interfere with chromogenic assays.
References
-
33742436
Douxfils J, Adcock DM, Bates SM, et al. 2021 update of the International Council for Standardization in Haematology recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2021;121(8):1008-1020.
-
Eliquis-summary of product characteristics
European Medicines Agency. Eliquis: summary of product characteristics. Accessed July 2021.
-
23999929
Mueck W, Stampfuss J, Kubitza D, et al. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53(1):1-16.
29193737
Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16(2):209-219.
29433148
Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437-450.


 Feedback
Feedback