Electromagnetic Mechanical Clot Detection / Chromogenic Assay
Lupus anticoagulant (LA) is an antiphospholipid antibody (aPL) autoantibody that binds to phospholipid-binding proteins, beta-2 glycoprotein I, and prothrombin, leading to a prothrombotic state. LA is associated with a range of adverse medical events, such as thrombosis and loss of pregnancy. LA can be transient (eg, due to certain medications or infections) and may not show clinical symptoms in all cases. No single test currently exists for the detection of LA; instead, a combination of clot-based tests with appropriate reflexive steps is recommended. Persistently positive LA, anticardiolipin (aCL) IgG or IgM, or anti-beta-2 glycoprotein 1 (anti-β2GP1) IgG or IgM results (ie, consecutively positive on at least two occasions 12 weeks apart) fulfill the laboratory classification criteria for antiphospholipid syndrome (APS); they are more likely than transiently positive LA to be associated with clinical symptoms. , ,
This test can be used to aid in the evaluation of unexplained prolonged activated partial thromboplastin time (aPTT) or for patients with a significant probability of having APS. To assess for APS, order the Lupus Anticoagulant Reflex Panel (3017009) with Cardiolipin Antibodies, IgG and IgM (0099344) and Beta-2 Glycoprotein 1 Antibodies, IgG and IgM (0050321) or consider the Antiphospholipid Syndrome Reflex Panel (3017157).
Test Description
Anticoagulant Neutralization
Activated charcoal-based direct oral anticoagulant (DOAC) neutralizing compounds (eg, DOAC-Stop) and heparinase (eg, Hepzyme) are included in the panel in cases where screening assays suggest the presence of anticoagulant medication. However, if clinically feasible, LA testing should be performed before starting anticoagulation therapy or after its completion.
| Anticoagulant | Comments |
|---|---|
| Direct Xa Inhibitors | DOAC-Stop neutralizes oral direct Xa inhibitors (rivaroxaban, apixaban, edoxaban) at concentrations up to 500 ng/mL It is recommended to wait 48-72 hrs after discontinuing an oral direct Xa inhibitor before drawing samples for LA testing |
| DTIs | DOAC-Stop neutralizes the oral DTI dabigatran and may neutralize parenteral DTIs (argatroban and hirudin analogues) It is recommended to wait 4-8 hrs after discontinuing parenteral DTI infusion before drawing samples for LA testing It is recommended to wait 48-72 hrs after discontinuing an oral DTI before drawing samples for LA testing |
| LMWH | Hepzyme neutralizes LMWH but may be less effective compared with its neutralization of UFH It is recommended to wait 24-48 hrs after discontinuing LMWH injections before drawing samples for LA testing |
| UFH | Hepzyme neutralizes typical therapeutic concentrations (≤2 units/mL) of UFH. Higher concentrations of UFH will not be completely neutralized It is recommended to wait 4-8 hrs after discontinuing a UFH infusion before drawing samples for LA testing |
| DTI, direct thrombin inhibitor; LMWH, low molecular weight heparin; UFH, unfractionated heparin | |
Reflex Pattern
ARUP’s Lupus Anticoagulant (LA) Reflex Panel always includes prothrombin time to evaluate for preanalytic issues. aPTT and dilute Russell's viper venom time [dRVVT] are always performed to screen for LA. If one or both screening assays are prolonged, then reflex testing for anticoagulant identification, mixing, and confirmation will be performed as follows:
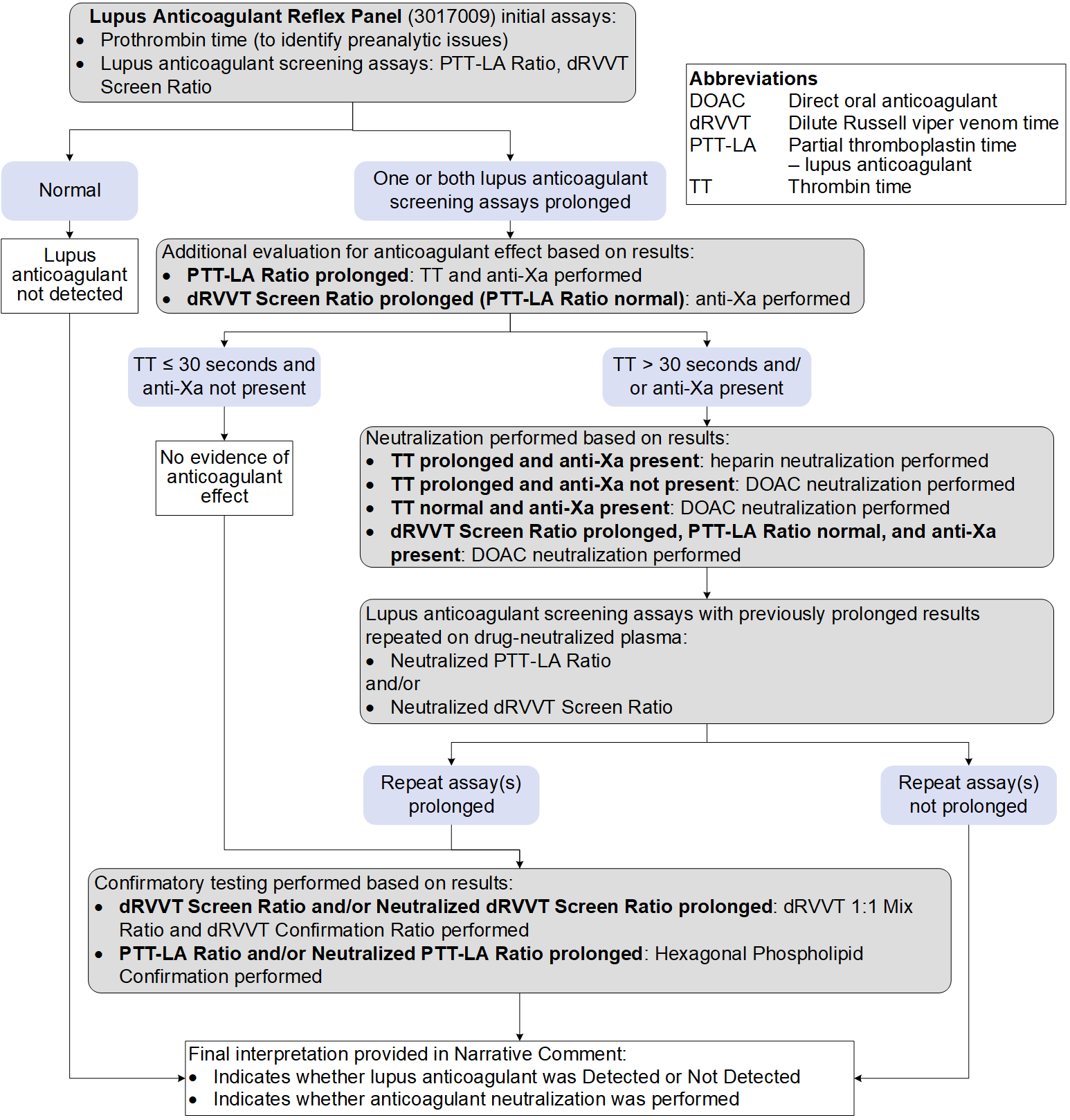
Test Interpretation
Results
| Panel Component | Result If Performed (Reported As) | Reference Interval |
|---|---|---|
| Prothrombin time | Time in seconds | 12.0-15.5 s |
| PTT-LA ratio | Ratio | ≤1.20 |
| dRVVT screen ratio | Ratio | ≤1.20 |
| Anti-Xa qualitative interpretation | Present or not present | Not present |
| Thrombin time | Time in seconds | ≤19.5 s |
| Anticoagulant medication neutralization | DOAC-Stop or Hepzyme | Not performed |
| Neutralized PTT-LA ratio | Ratio | ≤1.20 |
| Neutralized dRVVT screen ratio | Ratio | ≤1.20 |
| dRVVT 1:1 mix ratio | Ratio | ≤1.20 |
| dRVVT confirmation ratio | Ratio | ≤1.20 |
| Hexagonal phospholipid confirmation | Time in seconds (delta) | ≤7.9 s |
| LA interpretation | LA detected or LA not detected with a narrative comment | N/A |
Limitations
- Results should be interpreted within the context of a patient’s complete clinical picture.
- The following factors may result in test interference or spurious results:
- Acute phase reactions due to pregnancy, malignancy, inflammatory or infectious states, or trauma
- Liver disease
- Anticoagulant medications in concentrations exceeding the capacity of neutralizing reagents (heparins, DOACs)
- Warfarin effect
- Residual platelets (≥10,000 platelets/uL) in the plasma sample
- Specific factor antibodies directed against clotting factors involved in the intrinsic or common pathways
References
-
33462974
Devreese KMJ, de Groot PG, de Laat B, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. 2020;18(11):2828-2839.
-
32619349
Tripodi A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18(7):1569-1575.
-
37640450
Barbhaiya M, Zuily S, Naden R, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. 2023;82:1258-1270.


 Feedback
Feedback