Semi-Quantitative Cell-Based Indirect Fluorescent Antibody / Qualitative Immunoblot / Semi-Quantitative Indirect Fluorescent Antibody (IFA)
Semi-Quantitative Indirect Fluorescent Antibody (IFA)/Qualitative Immunoblot/Semi-Quantitative Cell-Based Indirect Fluorescent Antibody
Paraneoplastic neurologic syndromes are rare disorders that occur due to the remote effects of tumors. Antibodies associated with these conditions may be present in the serum or cerebrospinal fluid (CSF) and can serve as useful markers of disease.
Disease Overview
Paraneoplastic neurologic syndromes are associated with a number of tumor types, including small cell lung cancer, thymoma, neuroblastoma, Hodgkin lymphoma, and ovarian, breast, and testicular tumors. Researchers believe that paraneoplastic neurologic syndromes are caused by cancer-fighting components of the immune system, particularly antibodies and T cells. Instead of attacking only cancer cells, these immune agents also attack the normal cells of the nervous system and cause neurologic disorders. Paraneoplastic neurologic syndromes may present with multiple clinical manifestations (e.g., encephalitis, autonomic failure, peripheral neuropathy, cerebellar ataxia, and visual complaints).
Certain well-characterized antineural antibodies (e.g., Hu, Ri, and Yo) are strongly associated with cancer and provide valuable information about which malignancies might need to be screened for in a patient. The detection of an antibody can also guide treatment decisions.
For more information about laboratory testing for paraneoplastic syndromes and other autoimmune neurologic diseases, including detailed information about panel test selection, refer to the ARUP Consult Autoimmune Neurologic Diseases - Antineural Antibody Testing topic.
Test Description
ARUP’s serum and CSF paraneoplastic reflexive panels can be used to aid in the diagnosis of paraneoplastic neurologic syndromes. Testing for the presence of antineural antibodies in both serum and CSF is recommended in most situations.
These panels only evaluate for the presence of high-risk paraneoplastic antibodies, independent of neurologic phenotype. As such, they do not include many clinically relevant antibodies that are not highly associated with cancer. Targeted phenotype-specific panels are preferred for the evaluation of autoimmune neurologic disease; strongly consider choosing one of these panels:
| ARUP Panel | Test Code | |
|---|---|---|
| Serum | CSF | |
| Autoimmune Encephalopathy/Dementia Panel | 3006201 | 3006202 |
| Autoimmune Epilepsy Panel | 3006204 | 3006205 |
| Autoimmune Movement Disorder Panel | 3018964 | 3018966 |
| Autoimmune Myelopathy Panel | 3006208 | 3006209 |
| Autoimmune Dysautonomia Panel | 3006203 | — |
| Autoimmune Pediatric CNS Disorders | 3006210 | 3006211 |
| Autoimmune Stiff-Person Disorders | 3006234 | 3006235 |
Antibodies Tested and Methodology
| Autoantibody Marker | Method | Individual Autoantibody or Focused Panel Test Code | |
|---|---|---|---|
| Serum | CSF | ||
| Amphiphysin Ab, IgG | IB | 2008893 | 3004510 |
| ANNA-1 (Hu) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| ANNA-2 (Ri) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| CV2 (CRMP-5) Ab, IgG | CBA-IFA, reflex titer | 3016999 | 3017001 |
| Ma2/Ta Ab, IgG | IB | 3017441 | 3017440 |
| PCCA-1 (Yo) | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| PCCA-Tr/DNER | IFA, reflex IB, reflex titer | 2007961 | 2010841 |
| SOX1 (AGNA) Ab, IgG | IB | 3002885 | 3002886 |
| Ab, antibody; AGNA, antiglial nuclear antibody; ANNA-1, antineuronal nuclear antibody type 1; ANNA-2, antineuronal nuclear antibody type 2; CBA, cell-binding assay/cell-based assay; CRMP-5, collapsin response-mediator protein 5; DNER, Delta/notch-like epidermal growth factor-related receptor; IB, immunoblot; IFA, indirect immunofluorescence assay; Ig, immunoglobulin; PCCA-1, Purkinje cell cytoplasmic antibody type 1; PCCA-Tr, Purkinje cell cytoplasmic antibody type Tr; SOX1, SRY-box transcription factor 1 | |||
Reflex Patterns
Paraneoplastic Reflexive Panel Serum (3002929) and CSF (3004517): Reflex Patterns
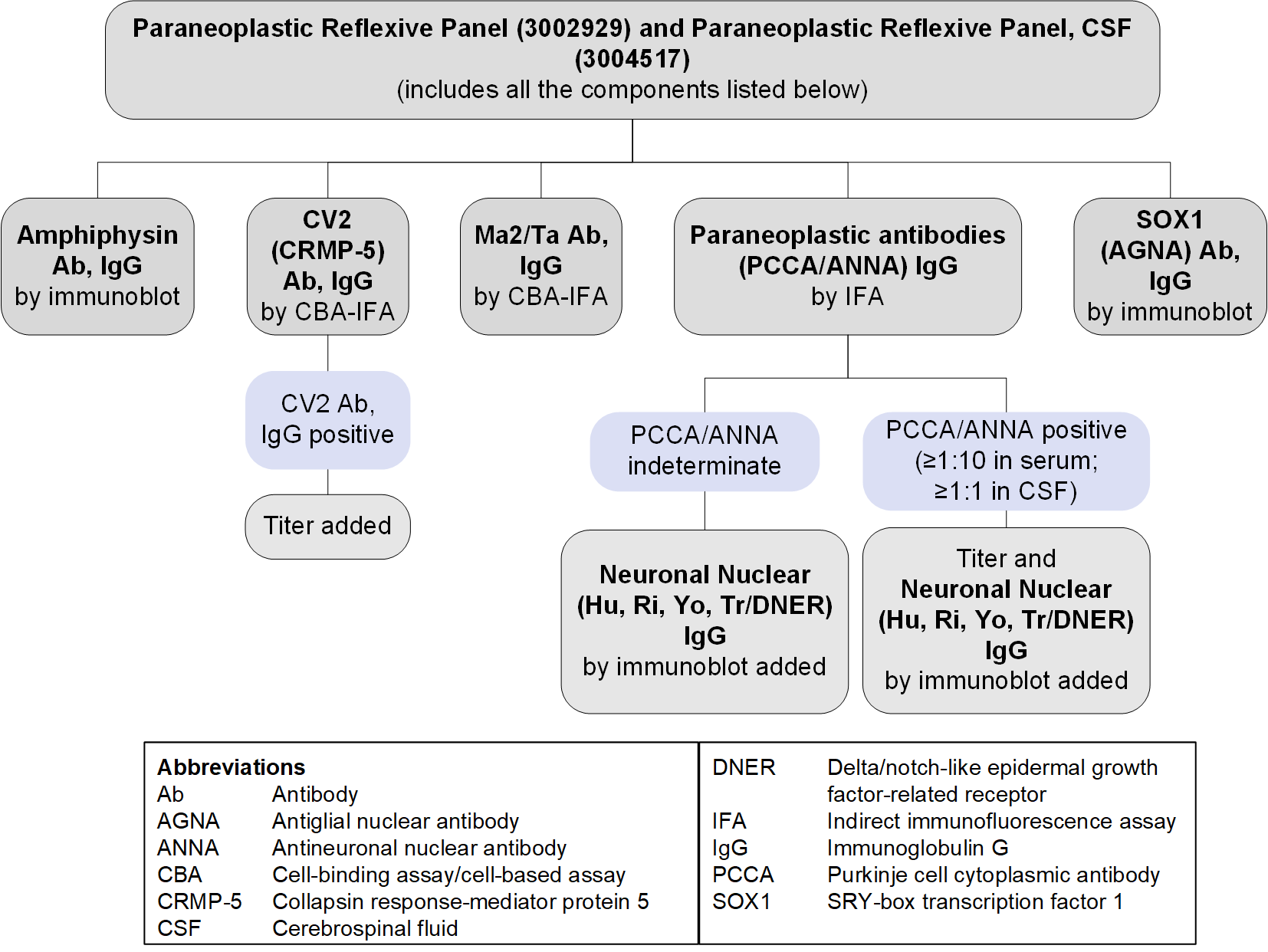
Limitations
These tests evaluate only for the presence of high-risk paraneoplastic antibodies. As testing for newly described antibodies becomes available and their clinical relevance is established, these panels may evolve to reflect these discoveries.
Test Interpretation
Results
Results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected May support a diagnosis of a paraneoplastic neurologic syndrome
|
| Negative | No autoantibodies detected The diagnosis of a paraneoplastic neurologic syndrome is not excluded |
References
-
20880069
Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18(1):19-e3.
-
16549814
de Beukelaar JW, Sillevis Smitt PA. Managing paraneoplastic neurological disorders. Oncologist. 2006;11(3):292-305.


 Feedback
Feedback