Medical Experts
Doyle

Straseski

Transgender and gender-diverse individuals are those who do not identify with the sex they were assigned at birth. Some, but not all, transgender and gender-diverse individuals seek gender-affirming interventions to help align physical characteristics with their gender identity. Hormone therapy is one such intervention, and laboratory testing plays an important role in establishing a safe and effective hormone regimen. Other medical care for transgender and gender-diverse individuals, including preventive care screening, should be personalized based on each individual’s biology and preferences.
This ARUP Consult topic summarizes currently established recommendations for adult transgender males and adult transgender females receiving hormone therapy, with the understanding that this field of medicine continues to evolve. The following resources provide more detailed recommendations, including recommendations for youth and adolescents:
- Endocrine Society Clinical Practice Guideline: Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons
- University of California, San Francisco (UCSF) Transgender Care and Treatment Guidelines: Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People
- World Professional Association for Transgender Health (WPATH): Standards of Care for the Health of Transgender and Gender Diverse People, Version 8
For definitions of terms as they are used in this topic, refer to the Glossary section.
Quick Answers for Clinicians
Testosterone concentrations, in the case of transgender males, and testosterone and estradiol concentrations, in the case of transgender females, should be measured regularly during and after gender-affirming hormone therapy. , , Testing by tandem mass spectrometry is more specific and accurate than immunoassay across a wider range of hormone concentrations; this method is preferable if low concentrations of a specific hormone are expected (e.g., at baseline or at initiation of some gender-affirming hormone therapies). Testing by immunoassay is accurate at higher hormone concentrations and may be more cost-effective when clinically appropriate (e.g., for monitoring long-term gender-affirming hormone therapy use).
Refer to the Monitoring of Gender-Affirming Hormone Therapy testing algorithm for a visual representation. Laboratory tests should also be used to evaluate other secondary health risks of hormone therapy, such as metabolic and electrolyte imbalances.
The typical goals of gender-affirming hormone therapy are to suppress endogenous sex steroids and maintain exogenous sex steroids within the reference interval of the affirmed gender or within a range that achieves the desired outcome. , Broadly applicable and well-studied reference intervals specific for transgender patients have not been established. In many cases, the applicable reference intervals for the affirmed gender may provide an initial goal, but results should be interpreted with caution and in the context of the clinical situation. The Endocrine Society Clinical Practice Guideline and UCSF Transgender Care and Treatment Guidelines provide more specific guidance.
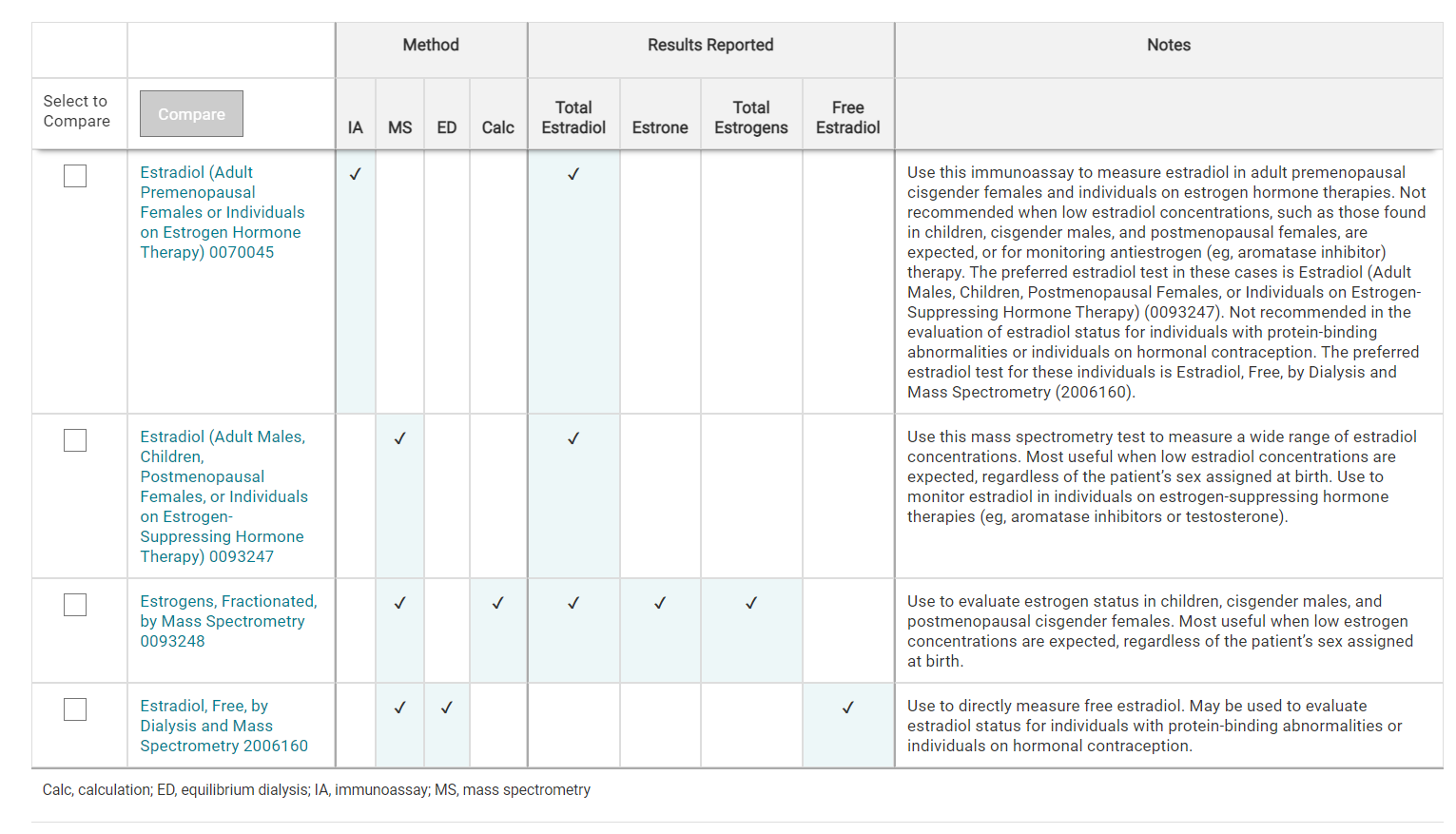
For comparisons of ARUP estrogen and testosterone tests, refer to the Estrogen Tests Comparison and Testosterone Tests Comparison tables.
Treatment Monitoring
Youth and Adolescents
For recommendations for adolescents, refer to the Endocrine Society Clinical Practice Guideline. The UCSF Transgender Care and Treatment Guidelines and WPATH Standards of Care also offer guidance for children and adolescents.
Adult Transgender Males on Testosterone Hormone Therapy
Monitoring of Hormone Therapy
The Endocrine Society Clinical Practice Guideline and WPATH Standards of Care recommend that serum testosterone concentrations be measured every 3 months until serum testosterone is within the reference interval for an adult cisgender male. The UCSF Transgender Care and Treatment Guidelines m suggest that annual monitoring is reasonable after reaching the desired range. The WPATH Standards of Care suggest monitoring once or twice a year after the first year of hormone therapy. The timing of measurements depends on the type of therapy used.
- For testosterone enanthate/cypionate injections, the suggested serum testosterone concentration is 400-700 ng/dL; measurements can be taken either midway between injections or at peak and trough concentrations. ,
- For parenteral testosterone undecanoate, the dose interval should be increased if testosterone concentrations are <400 ng/dL. Measurements should be taken right before the next injection (i.e., at trough concentrations). ,
- For transdermal testosterone, measurements should be taken no sooner than after 1 week of daily application and at least 2 hours after application. ,
Testing can be performed using either tandem mass spectrometry or immunoassay. Tandem mass spectrometry is more specific and accurate across a wider range of concentrations and is preferable if low testosterone concentrations are expected (e.g., at baseline or at initiation of some gender-affirming hormone therapies). Testing by immunoassay is accurate at higher hormone concentrations and may be more cost-effective when clinically appropriate (e.g., for monitoring long-term gender-affirming hormone therapy use).
Free or bioavailable testosterone tests may provide supportive information for patients with unexpected responses to hormone therapy. Free and bioavailable testosterone concentrations are typically estimated using calculations that include testosterone, albumin, and/or sex hormone-binding globulin (SHBG) measurements. Tests that measure SHBG may be useful in individuals with protein-binding abnormalities. Equilibrium dialysis may be used for the direct measurement of free testosterone, but it is not frequently used as a first-line test for monitoring of hormone therapy. Calculated methods of determining free testosterone concentrations are sufficient in most clinical circumstances.
For a visual overview of testing associated with this topic, please refer to the Monitoring of Gender-Affirming Hormone Therapy testing algorithm.
For a comparison of ARUP testosterone tests, refer to the Testosterone Tests Comparison table.
Other Testing
In addition to serum testosterone measurements, the Endocrine Society Clinical Practice Guideline recommends the following tests or assessments. Additional tests may also be recommended by other organizations.
- Clinical assessment of masculinization and monitoring for adverse reactions should be performed every 3 months in the first year, then once or twice annually.
- Hematocrit or hemoglobin should be tested before beginning gender-affirming hormone therapy, then every 3 months during the first year of therapy, and then once or twice annually. ,
Adult Transgender Females on Estrogen Hormone Therapy and/or Testosterone-Suppressing Hormone Therapy
Monitoring of Hormone Therapy
The Endocrine Society Clinical Practice Guideline recommends that serum testosterone and serum estradiol concentrations be measured every 3 months. These and other guidelines suggest a goal of testosterone concentrations <50 ng/dL and serum estradiol concentrations within the peak physiologic range of 100-200 pg/mL. , The UCSF Transgender Care and Treatment Guidelines recommend measurement of estradiol at 3 months, 6 months, and then as needed, and recommend total testosterone and SHBG/albumin (to calculate bioavailable testosterone) at 3 months, 6 months, and 1 year.
Testing can be performed using either tandem mass spectrometry or immunoassay. Tandem mass spectrometry is more specific and accurate across a wider range of concentrations and is preferable if low hormone concentrations are expected (e.g., at baseline or at initiation of some gender-affirming hormone therapies). Testing by immunoassay is accurate at higher hormone concentrations and may be more cost-effective when clinically appropriate (e.g., for monitoring long-term gender-affirming hormone therapy use). Equilibrium dialysis may be used to directly measure free estradiol in individuals on hormonal contraception and/or with protein-binding abnormalities.
Free or bioavailable testosterone tests may provide supportive information for patients with unexpected responses to hormone therapy. Free and bioavailable testosterone concentrations are typically estimated using calculations that include testosterone, albumin, and/or SHBG measurements. Tests that measure SHBG may be useful in individuals who have protein-binding abnormalities or are taking estrogen supplementation.
For a visual overview of testing associated with this topic, please refer to the Monitoring of Gender-Affirming Hormone Therapy testing algorithm.
For comparisons of ARUP estrogen and testosterone tests, refer to the Estrogen Tests Comparison and Testosterone Tests Comparison tables.
Other Testing
In addition to serum hormone measurements, the Endocrine Society Clinical Practice Guideline recommends the following tests or assessments. Additional tests may also be recommended by other organizations.
- Clinical assessment of feminization and monitoring for adverse reactions should be performed every 3 months in the first year, then once or twice annually.
- Serum electrolyte concentrations (particularly potassium concentrations) should be measured every 3 months in the first year, then annually in individuals on spironolactone. , An assessment of renal function (particularly creatinine) is also recommended. ,
- The Endocrine Society Clinical Practice Guideline recommends the periodic monitoring of prolactin concentrations in transgender individuals treated with estrogens. The WPATH Standards of Care suggest that, in consideration of accessibility and costs, prolactin monitoring should be based on clinical judgment informed by an individual’s specific treatment regimen and symptoms.
Preventive Care Screening
The following preventive screenings for applicable health risks are recommended as part of treatment monitoring. Other screenings may also be recommended.
- Osteoporosis screening should be performed in individuals who stop testosterone treatment or otherwise develop risk for bone loss.
- Routine cancer screening should be performed for all tissues present. For example, cervical cancer screening, if cervical tissue is present, should be performed according to the recommendations from the American College of Obstetricians and Gynecologists. , Breast cancer screenings for individuals who retain breast tissue should follow recommendations for cisgender women. Alternative breast cancer screening strategies may be needed for individuals who have had a mastectomy. Screening should be performed according to individual risk for prostatic disease and prostate cancer.
- Cardiovascular disease risk should be assessed in transgender individuals treated with hormones. This risk assessment should take into consideration an individual’s hormone regimen, duration of hormone use, age at which therapy was started, and serum hormone concentrations.
Glossary
This glossary is presented solely to define terms as they are used in this ARUP Consult topic and the following resources:
- Monitoring of Gender-Affirming Hormone Therapy testing algorithm
- Testosterone Tests Comparison table
- Estrogen Tests Comparison table
This glossary is intended to facilitate appropriate laboratory test selection for individuals who are on gender-affirming hormone therapy. The terminology in this field changes rapidly, and these terms may be used differently by others and in different contexts.
Sex and Gender Terms
Cisgender female: An individual with a female gender identity who was assigned a female sex at birth.
Cisgender male: An individual with a male gender identity who was assigned a male sex at birth.
Gender: Nonbiologic characteristics (e.g., behaviors, roles, social attributes) that a culture associates with an individual’s sex. Gender identity is defined as an individual’s internal sense of their gender. , ,
Gender dysphoria: Distress associated with discordance between an individual’s gender identity and their sex assigned at birth. , , ,
Sex: A combination of biologic characteristics (e.g., chromosomes, genes, genitalia, gonads, hormones, secondary sex characteristics). Sex is often assigned at birth based on external genital anatomy. , ,
Transgender female: An individual with a female gender identity who was assigned a male sex at birth. , ,
Transgender male: An individual with a male gender identity who was assigned a female sex at birth. , ,
Hormone Therapy Terms
Estrogen hormone therapy: Hormone treatment intended to maintain exogenous estrogen within a desired range.
Estrogen-suppressing hormone therapy: Hormone treatment intended to suppress an individual’s endogenous estrogen to within a desired range. This may include aromatase inhibitor therapies.
Gender-affirming hormone therapy: Hormone treatment that is intended to help change an individual’s physical characteristics to best align with their gender identity. This may include therapies to suppress endogenous sex steroids and/or maintain exogenous sex steroids within a desired physiologic range.
Testosterone hormone therapy: Hormone treatment intended to maintain exogenous testosterone within a desired range.
Testosterone-suppressing hormone therapy: Hormone treatment intended to suppress an individual’s endogenous testosterone (or testosterone activity) to within a desired range. This may include antiandrogen therapies such as spironolactone and cyproterone.
ARUP Laboratory Tests
Use this immunoassay to monitor testosterone hormone therapies
Free or bioavailable testosterone measurements may provide supportive information
This test is not recommended when low testosterone concentrations, such as those found in children and cisgender females, are expected
Quantitative Electrochemiluminescent Immunoassay
Provides a calculated value for free testosterone concentration using total testosterone measured by immunoassay
May be used to monitor testosterone hormone therapies
This test is not recommended when low testosterone concentrations, such as those found in children and cisgender females, are expected
Quantitative Electrochemiluminescent Immunoassay/Calculation
Provides a calculated value for free testosterone concentration using total testosterone measured by immunoassay
May be used to monitor testosterone hormone therapies
This test is not recommended when low testosterone concentrations, such as those found in children and cisgender females, are expected
Quantitative Electrochemiluminescent Immunoassay/Calculation
Provides a calculated value for bioavailable testosterone concentration using total testosterone measured by immunoassay
May be used to monitor testosterone hormone therapies
This test is not recommended when low testosterone concentrations, such as those found in children and cisgender females, are expected
Quantitative Electrochemiluminescent Immunoassay/Calculation
Laboratory reference method for the direct measurement of free testosterone
These tests are not recommended when low testosterone concentrations, such as those found in children and cisgender females, are expected
Quantitative Equilibrium Dialysis (ED)/High Performance Liquid Chromatography-Tandem Mass Spectrometry
Quantitative Equilibrium Dialysis/High Performance Liquid Chromatography-Tandem Mass Spectrometry
Use this mass spectrometry test to measure a wide range of testosterone concentrations
Most useful when low concentrations are expected, regardless of the patient’s sex assigned at birth
Use to monitor testosterone-suppressing hormone therapies (e.g., antiandrogens or estrogens)
Free or bioavailable testosterone measurements may provide supportive information
Quantitative High Performance Liquid Chromatography-Tandem Mass Spectrometry
Provides a calculated value for free testosterone concentration using total testosterone measured by mass spectrometry
May be used monitor testosterone-suppressing hormone therapies (e.g., antiandrogens or estrogens)
Quantitative High Performance Liquid Chromatography-Tandem Mass Spectrometry / Electrochemiluminescent Immunoassay (ECLIA) / Calculation
Provides a calculated value for free testosterone concentration using total testosterone measured by mass spectrometry
May be used to monitor testosterone-suppressing hormone therapies (e.g., antiandrogens or estrogens)
Quantitative High Performance Liquid Chromatography-Tandem Mass Spectrometry/Electrochemiluminescent Immunoassay/Calculation
Provides a calculated value for bioavailable testosterone concentration using total testosterone measured by mass spectrometry
May be used to monitor testosterone-suppressing hormone therapies (e.g., antiandrogens or estrogens)
Quantitative High Performance Liquid Chromatography-Tandem Mass Spectrometry/Electrochemiluminescent Immunoassay (ECLIA)/Calculation
For a comparison of ARUP estrogen tests, refer to the Estrogen Tests Comparison table.
Use this immunoassay to measure estradiol in individuals on estrogen hormone therapies
This test is not recommended when low estradiol concentrations, such as those found in children, cisgender males, and postmenopausal females, are expected
Quantitative Chemiluminescent Immunoassay (CLIA)
Use this mass spectrometry test to measure a wide range of estradiol concentrations
Most useful when low estradiol concentrations are expected, regardless of the patient’s sex assigned at birth
Use to monitor estradiol in individuals on estrogen-suppressing hormone therapies (e.g., aromatase inhibitors or testosterone)
Quantitative High Performance Liquid Chromatography-Tandem Mass Spectrometry
Use to directly measure free estradiol
May be used to evaluate estradiol status for individuals with protein-binding abnormalities or individuals on hormonal contraception
Quantitative Equilibrium Dialysis (ED) / High Performance Liquid Chromatography-Tandem Mass Spectrometry
References
-
28945902
Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline [published corrections appear in J Clin Endocrinol Metab. 2018;103(2):699, and J Clin Endocrinol Metab. 2018;103(7):2758-2759]. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
-
Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People
Deutsch MB, ed. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. University of California, San Francisco, Transgender Care. Published Jun 2016; accessed Nov 2021.
-
36238954
Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(Suppl 1):S1-S259.
-
27661651
Committee on Practice Bulletins - Gynecology. Practice bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111‐e130.
-
Advancing effective communication-lesbian, gay, bisexual, and transgender
The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered care for the lesbian, gay, bisexual, and transgender (LGBT) community: a field guide. Published Oct 2011; accessed Feb 2022.
-
APA - Diagnostic And Statistical Manual Of Mental Disorders 5th ed
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.


 Cite this page
Cite this page Feedback
Feedback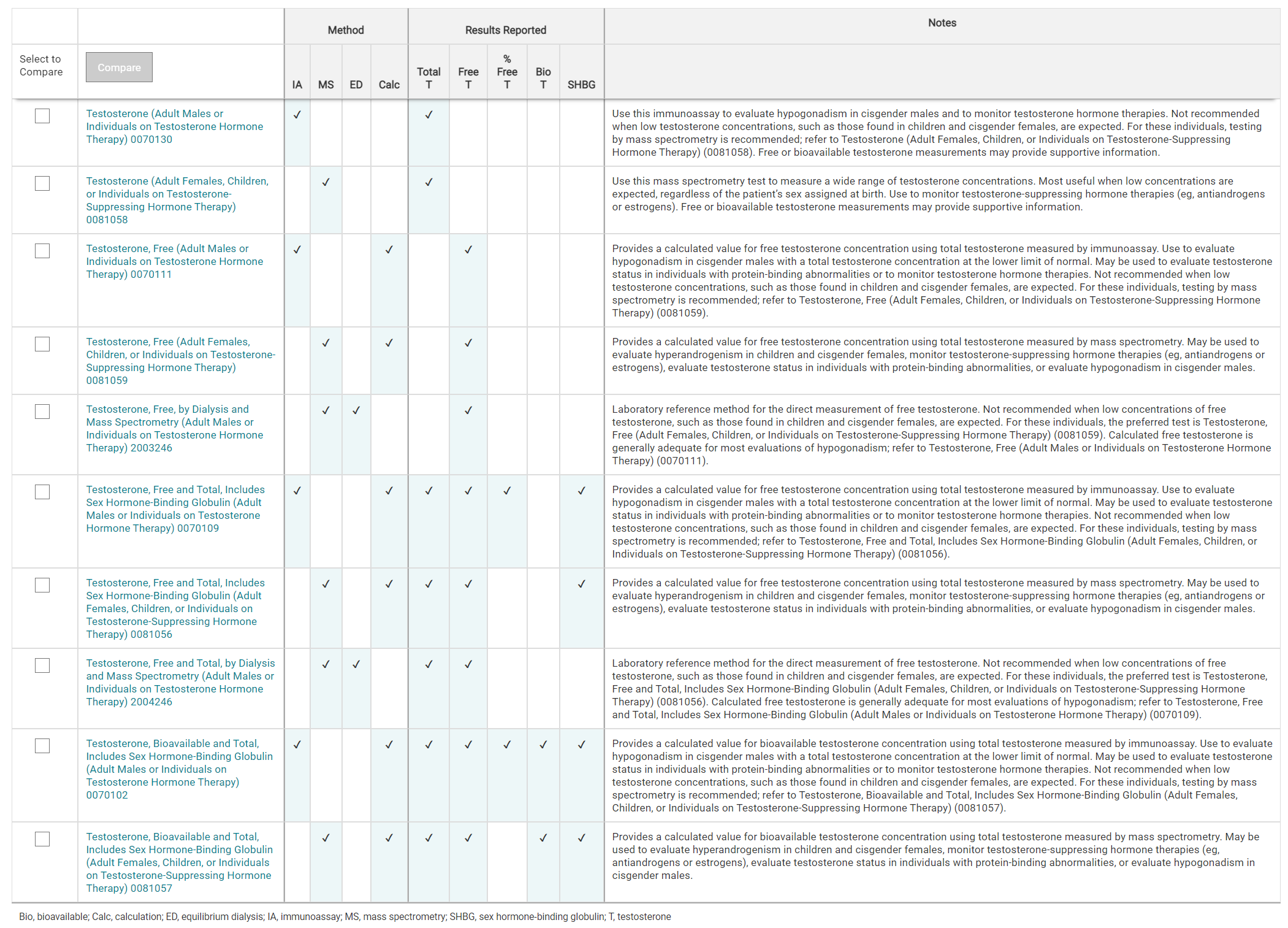
For a comparison of ARUP testosterone tests, refer to the Testosterone Tests Comparison table.