Quantitative Radioimmunoassay/Qualitative Radiobinding Assay/Semi-Quantitative Flow Cytometry/Semi-Quantitative Indirect Fluorescent Antibody
Autoimmune neuromuscular junction disorders are a broad range of rare, acquired conditions that affect the peripheral nervous system. Antibodies associated with these conditions can serve as useful markers of disease and help guide treatment.
Disease Overview
Autoimmune neuromuscular junction disorders generally develop subacutely and can progress rapidly. They are characterized by fatigable weakness which may affect ocular, bulbar, axial, and respiratory muscles. Antineural antibodies serve as useful markers of these diseases, and their detection may help establish a diagnosis, support treatment decisions, aid prognostication, serve as a prerequisite for enrollment in clinical trials, and guide the search for an associated malignancy.
For more information about laboratory testing for autoimmune neurologic diseases, including detailed information about panel test selection, refer to the ARUP Consult Autoimmune Neurologic Diseases - Antineural Antibody Testing topic. For more information about the testing strategy for myasthenia gravis, refer to the ARUP Consult Myasthenia Gravis - MG topic.
Test Description
ARUP’s Autoimmune Neuromuscular Junction Reflexive Panel can be used for the evaluation of suspected acquired autoimmune neuromuscular junction disorders.
This test is not recommended for the initial evaluation of myasthenia gravis. For more information on ARUP’s myasthenia gravis testing, refer to the Myasthenia Gravis Testing Test Fact Sheet.
Testing for individual autoantibodies is also available separately and can be used for long-term monitoring.
Antibodies Tested and Methodology
| Autoantibody Marker | Method | Individual Autoantibody Test Code |
|---|---|---|
| AChR Binding Ab, IgG | RIA | 0080009 |
| AChR Blocking Ab, IgG | Flow cytometry | 0099580 |
| AChR Modulating Ab, IgGa | Flow cytometry | 0099521 |
| CASPR2 Ab, IgGa | CBA-IFA, reflex titer | 2009452 |
| Ganglionic AChR Ab | RIA | 3003020 |
| LGI1 Ab, IgGa | CBA-IFA, reflex titer | 2009456 |
| N-type VGCC Ab, IgG | RIA | — |
| P/Q-type VGCC Ab, IgG | RIA | 0092628 |
| Striated Muscle Abs, IgG | IFA, reflex titer | 0050746 |
| Titin Ab, IgG | IFA | 2005636 |
| VGKC Ab, IgG | RIA | 2004890 |
aPerformed via reflex only, depending on the results of other autoantibody tests; refer to Reflex Pattern diagram. Ab, antibody; AChR, acetylcholine receptor; CASPR2, contactin-associated protein 2; CBA, cell-binding assay/cell-based assay; IFA, indirect immunofluorescence assay; Ig, immunoglobulin; LGI1, leucine-rich, glioma-inactivated protein 1; mGluR1, metabotropic glutamate receptor 1; MOG, myelin oligodendrocyte glycoprotein; NMDAR, N-methyl-D-aspartate receptor; PCCA, Purkinje cell cytoplasmic antibody; RIA, radioimmunoassay; VGCC, voltage-gated calcium channel; VGKC, voltage-gated potassium channel | ||
Reflex Patterns
Autoimmune Neuromuscular Junction Reflexive Panel (3003017): Reflex Pattern
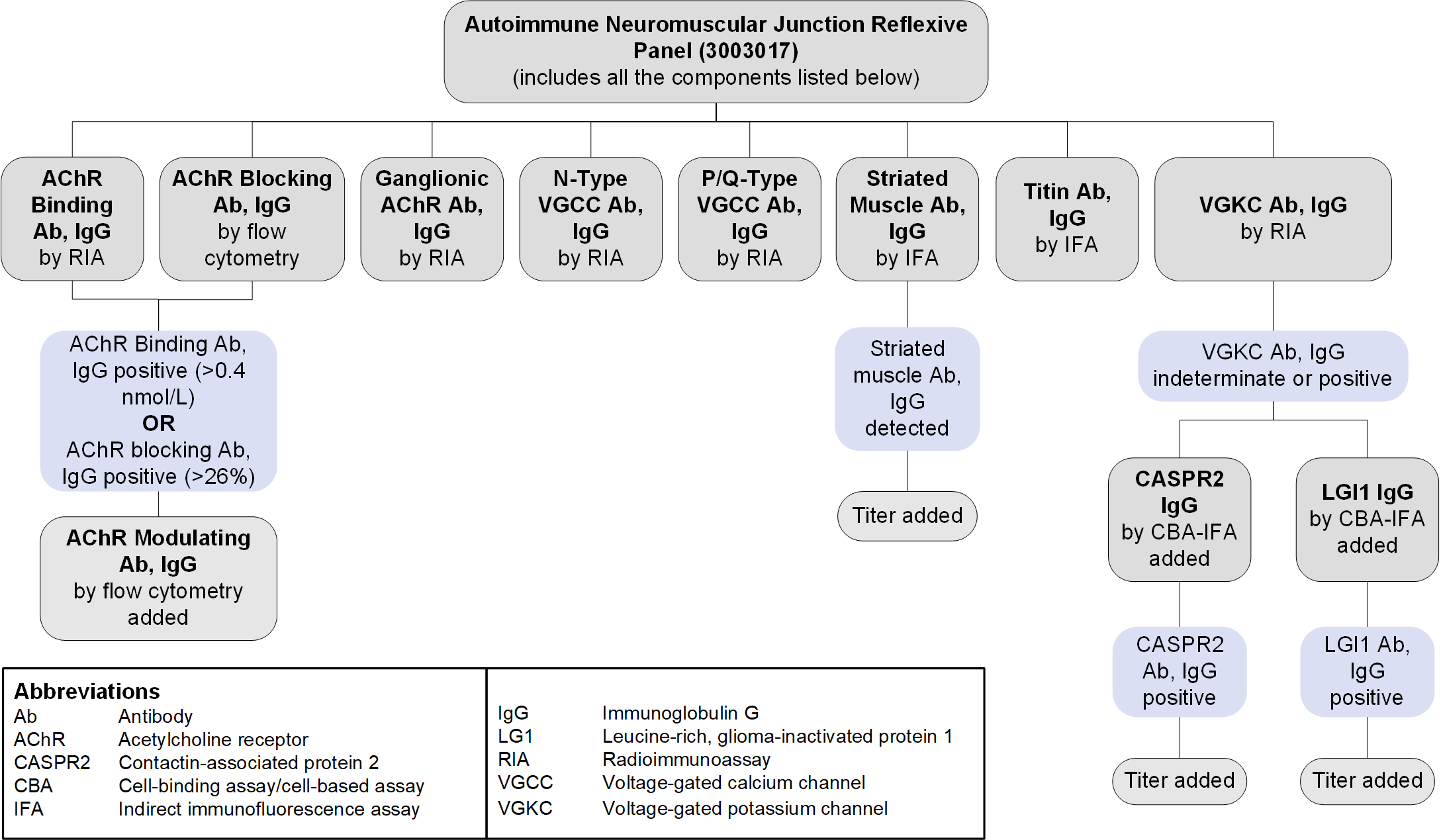
Limitations
These tests do not include all known antineural antibodies:
- Some antibodies are extremely rare or are of uncertain clinical significance.
- As testing for newly described antibodies becomes available and their clinical relevance is established, these panels will evolve to reflect these discoveries.
Test Interpretation
Results
Results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment.
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected May support a diagnosis of an autoimmune neuromuscular junction disorder |
| Negative | No autoantibodies detected A diagnosis of an autoimmune neuromuscular junction disorder is not excluded |
References
-
32117321
Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212.


 Feedback
Feedback