Immunohistochemistry (IHC)
Immunohistochemistry (IHC)
Testing for programmed death ligand 1 (PD-L1) expression by immunohistochemistry is used in certain types and stages of cancer (eg, cervical cancer, esophageal cancer, gastric cancer, head and neck cancer, non-small cell lung cancer, triple negative breast cancer, urothelial carcinoma) to identify patients most likely to benefit from treatment with immune checkpoint inhibitors such as PD-1 and CTLA-4 inhibitors. , , , , , , The U.S. Food and Drug Administration (FDA) divides PD-L1 testing into companion diagnostic tests (test results are required to use the drug) and complementary or codiagnostic tests (test results are not required but may be useful in treatment selection).
Test Selection
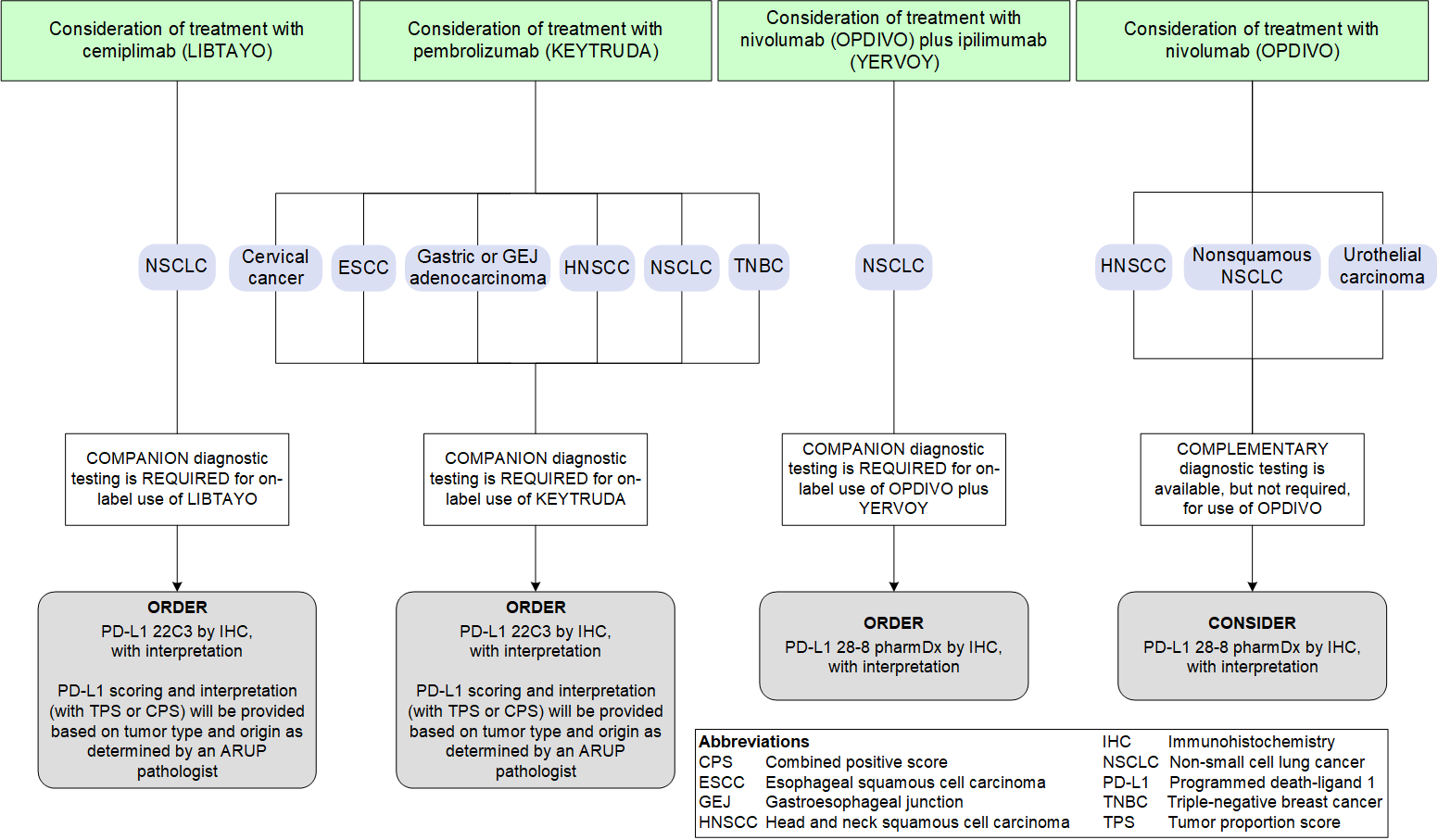
ARUP’s PD-L1 immunohistochemistry tests may be used as companion or complementary tests to identify potential treatment options in certain patients with cancers (refer to the FDA-Approved Indications for PD-L1 Testing diagram below). There are multiple interpretive scoring systems available for PD-L1 testing, including the combined positive score (CPS) and tumor proportion score (TPS). PD-L1 scoring and interpretation (with CPS or TPS) will be provided based on tumor type and origin as determined by an ARUP pathologist.
In brief, the TPS is based on the proportion of viable tumor cells that express PD-L1 to the total number of tumor cells (staining and nonstaining). The CPS is calculated similarly to the TPS, but the CPS includes PD-L1 expression in tumor-associated lymphocytes and macrophages in addition to the ratio of PD-L1-expressing tumor cells. For the 22C3 assay, either TPS or CPS can be used. For the 28-8 assay, TPS can be used.
The selection of which scoring system to use, as well as the interpretive cutoffs, depends on the test, tumor, and indications. Please refer to the full prescribing information for tumor-specific indications and expression level cutoff (FDA website and package insert).
FDA-Approved Indications for PD-L1 Testing
Test Overview: PD-L1 22C3 by Immunohistochemistry, with Interpretation
PD-L1 22C3 is used as a companion diagnostic test for KEYTRUDA or LIBTAYO based on primary tumor type. The type of scoring will be determined by an ARUP medical director based on the provided pathology report.
Tumor proportion score (TPS) interpretation will be provided for non-small cell lung cancer (NSCLC) specimens to aid in the prediction of response to pembrolizumab (KEYTRUDA) and cemiplimab (LIBTAYO) for certain patients with NSCLC.
Combined positive score (CPS) interpretation will be provided for gastric, esophageal, gastroesophageal junction (GEJ) adenocarcinoma, cervical carcinomas (squamous cell carcinomas and adenocarcinomas), esophageal squamous cell carcinoma (ESCC), head and neck squamous cell carcinoma (HNSCC), and triple-negative breast cancer (TNBC) specimens to aid in prediction of response to pembrolizumab (KEYTRUDA).
Other tumor types will be reported with TPS and an off-label disclaimer.
| Tumor Type | Treatment Considered | Score Reported | PD-L1 Expression Cutoff |
|---|---|---|---|
| Cervical cancer | Pembrolizumab | CPS | ≥1 |
| ESCC | Pembrolizumab | CPS | ≥10 |
| Gastric, esophageal, or GEJ adenocarcinoma | Pembrolizumab | CPS | ≥1 |
| HNSCC | Pembrolizumab | CPS | ≥1 |
| NSCLC | Cemiplimab | TPS | ≥50% |
| Pembrolizumab | TPS | ≥1% | |
| TNBC | Pembrolizumab | CPS | ≥10 |
| Other tumor types | Any | TPS | Reported with an off-label disclaimer |
Test Overview: PD-L1 28-8 pharmDx by Immunohistochemistry with Interpretation, nivolumab (OPDIVO)
PD-L1 28-8 is an FDA-approved companion diagnostic testing to aid in predicting a response to nivolumab (OPDIVO) in combination with ipilimumab (YERVOY) for certain patients with non-small cell lung carcinoma.
PD-L1 28-8 is also an FDA-approved complementary codiagnostic test that may aid in predicting response to nivolumab (OPDIVO) treatment for patients with nonsquamous non-small cell lung cancer (NSCLC), urothelial carcinoma, or head and neck squamous cell carcinoma (HNSCC).
| Tumor Type | Treatment Considered | Score Reported | PD-L1 Expression Cut-Off |
|---|---|---|---|
| NSCLC | Combination therapy with nivolumab plus ipilimumab | TPS | ≥1% |
| Nonsquamous NSCLC, HNSCC, UC | Nivolumab | TPS | Test results are NOT required for drug use |
| Other tumor types | Nivolumab with or without ipilimumab | TPS | Reported with an off-label disclaimer |
Limitations
ARUP’s PD-L1 tests were validated on nondecalcified, formalin-fixed (10 percent neutral buffered formalin), and paraffin-embedded tissue specimens.
The specimen submitted for testing must contain at least 100 viable, invasive tumor cells to be considered adequate for evaluation.
References
-
NCCN - head and neck cancers v4.2024
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: head and neck cancers. Version 4.2024. Updated May 2024; accessed May 2024.
-
NCCN - non-small cell lung cancer v5.2024
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: non-small cell lung cancer. Version 5.2024. Updated Apr 2024; accessed May 2024.
-
NCCN - breast cancer v2.2024
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: breast cancer. Version 2.2024. Updated Mar 2024; accessed May 2024.
-
NCCN - cervical cancer v3.2024
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: cervical cancer. Version 3.2024. Updated May 2024; accessed May 2024.
-
NCCN - esophageal-esophagogastric junction cancers v3.2024
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: esophageal and esophagogastric junction cancers. Version 3.2024. Updated Apr 2024; accessed May 2024.
-
NCCN - gastric cancer v2.2024
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: gastric cancer. Version 2.2024. Updated May 2024; accessed May 2024.
-
NCCN - bladder cancer v4.2024
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: bladder cancer. Version 4.2024. Updated May 2024; accessed May 2024.
-
FDA - list of cleared-approved companion diagnostic devises
U.S. Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Updated Oct 2024; accessed Nov 2024.


 Feedback
Feedback