Semi-Quantitative Cell-Based Indirect Fluorescent Antibody
Autoimmune central nervous system (CNS) demyelinating diseases include acute disseminated encephalomyelitis (ADEM), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), and neuromyelitis optica spectrum disorder (NMOSD). Antibodies associated with these conditions can serve as useful markers of disease.
Disease Overview
Autoimmune CNS demyelinating diseases, including ADEM, MOGAD, and NMOSD, are inflammatory disorders in which the dysregulated immune system targets antigens within the CNS. The most common manifestations of these diseases are optic neuritis, acute myelitis, or encephalopathy. Antineural antibodies serve as useful markers of these diseases, and their detection may help establish a diagnosis, support treatment decisions, aid prognostication, and serve as a prerequisite for enrollment in clinical trials. For more information about the testing strategy for NMOSD, refer to the ARUP Consult Neuromyelitis Optica Spectrum Disorders topic.
Multiple sclerosis (MS) is also an inflammatory demyelinating disease, but there are no specific antibody markers for this disease. For more information about the testing strategy for MS, refer to the ARUP Consult Multiple Sclerosis topic.
Test Description
ARUP’s Autoimmune CNS Demyelinating Disease Reflexive Panel can be used for the evaluation of suspected autoimmune CNS demyelinating diseases, including ADEM, MOGAD, and NMOSD. This test is not intended for the evaluation of MS; for more information about appropriate testing for MS, refer to the ARUP Consult Multiple Sclerosis topic.
This panel includes antibodies associated with autoimmune CNS demyelinating disease. If there is subacute onset of progressive bilateral vision loss and concern for a paraneoplastic autoimmune etiology, consider the Autoimmune Vision Loss Reflexive Panel, which includes recoverin and CV2 antibodies. To compare these panels and the antibodies included, refer to ARUP Antineural Antibody Testing for Autoimmune Neurologic Disease page.
Testing for individual autoantibodies is also available separately and can be used for long-term monitoring.
Antibodies Tested and Methodology
| Autoantibody Marker | Method | Individual Autoantibody Test Code |
|---|---|---|
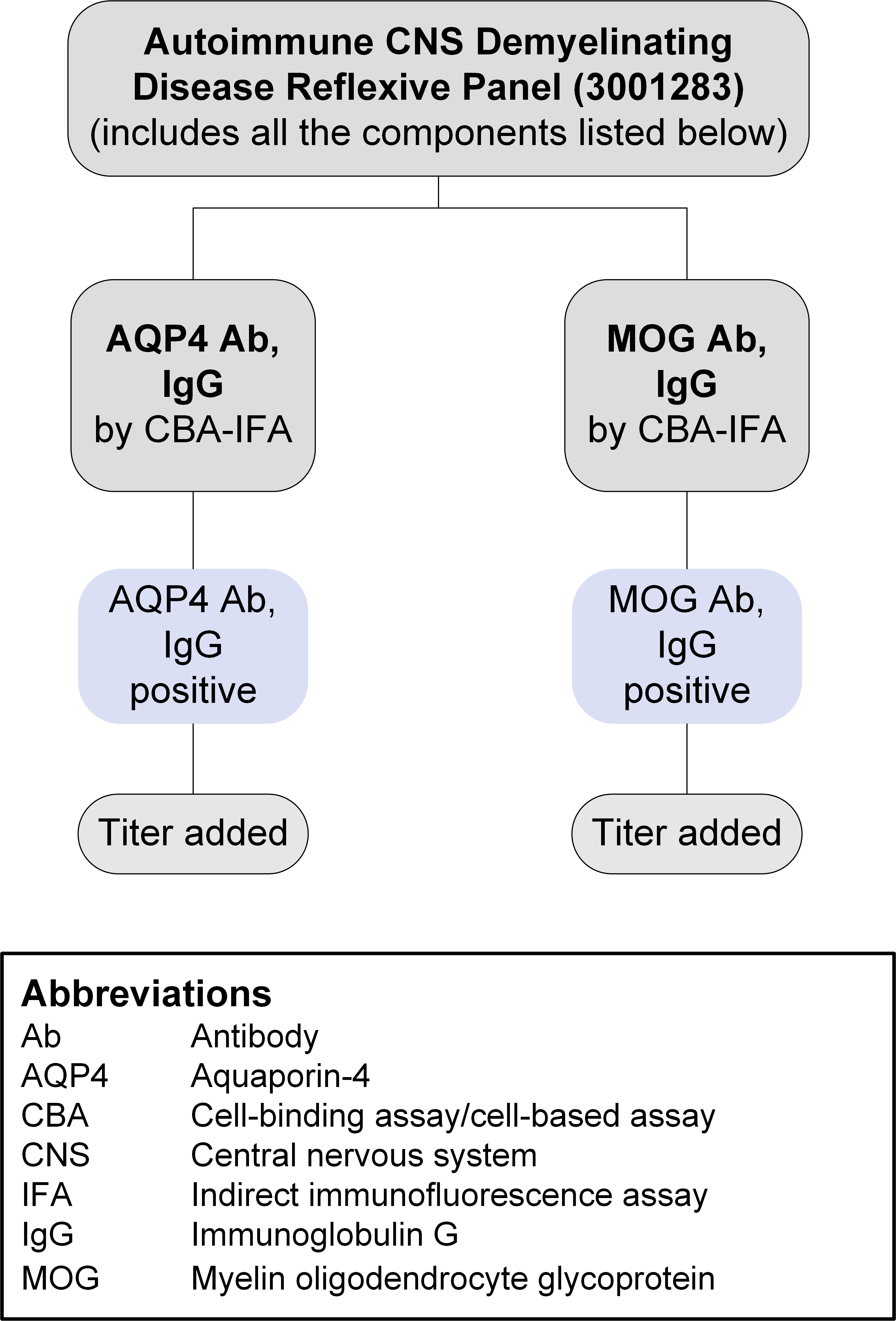
| AQP4 Ab, IgG | CBA-IFA, reflex titer | 2013320 |
| MOG Ab, IgG | CBA-IFA, reflex titer | 3001277 |
| Ab, antibody; AQP4, aquaporin-4; CBA, cell-binding assay/cell-based assay; IFA, indirect immunofluorescence assay; Ig, immunoglobulin; MOG, myelin oligodendrocyte glycoprotein | ||
Reflex Patterns
Autoimmune CNS Demyelinating Disease Reflexive Panel (3001283): Reflex Pattern
Limitations
This test does not include all known antineural antibodies. Patients may present with a clinical autoimmune CNS demyelinating disease but be negative for both MOG and AQP4 antibodies. Future studies are needed to understand whether these double negative patients have an as-yet undefined antineural antibody.
Test Interpretation
Results
Results must be interpreted in the clinical context of the individual patient; test results (positive or negative) should not supersede clinical judgment. This test is performed using a fixed CBA. Rare cases have been reported of patients testing negative using a fixed CBA, but positive using a live CBA. , If results are negative and there is a high suspicion for autoimmune CNS demyelinating disease, contact your laboratory and consider retesting by another method. At low titers (<1:40), the specificity of this assay decreases. ,
| Result | Interpretation |
|---|---|
| Positive for ≥1 autoantibodies | Autoantibody(ies) detected May support a diagnosis of an autoimmune CNS demyelinating disease |
| Negative | No autoantibodies detected A diagnosis of an autoimmune CNS demyelinating disease is not excluded |
References
-
35785363
Sechi E, Cacciaguerra L, Chen JJ, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): a review of clinical and MRI features, diagnosis, and management. Front Neurol. 2022;13:885218.
-
30728305
Waters PJ, Komorowski L, Woodhall M, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology. 2019;92(11):e1250-e1255.
-
32024795
Reindl M, Schanda K, Woodhall M, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e674.
-
35320765
Levy M, Yeh EA, Hawkes CH, et al. Implications of low-titer MOG antibodies. Mult Scler Relat Disord. 2022;59:103746.
-
35669883
Alkabie S, Budhram A. Testing for antibodies against aquaporin-4 and myelin oligodendrocyte glycoprotein in the diagnosis of patients with suspected autoimmune myelopathy. Front Neurol. 2022;13:912050.


 Feedback
Feedback