Supplemental Content
Medical Experts
SmithT

Multiple sclerosis (MS) is a chronic autoimmune disease characterized by inflammation and central nervous system (CNS) demyelination. MS generally presents with recurrent, subacute, focal neurologic symptoms, although symptoms can vary widely and may include monocular vision loss and limb sensory loss. Diagnosis of MS can be challenging because of variable disease presentation and significant clinical overlap with diseases such as neuromyelitis optica spectrum disorders (NMOSDs) and myelin oligodendrocyte glycoprotein-associated disease (MOGAD). Treatment of MS differs from that of other diseases, and using MS medications to treat other immune-mediated neurologic diseases can exacerbate them. Therefore, accurate diagnosis is critical to providing proper medical management.
Diagnosis of MS relies primarily on clinical evaluation and imaging studies such as magnetic resonance imaging (MRI), particularly in patients who present with typical disease progression. Laboratory evaluation such as cerebrospinal fluid (CSF) studies can support a diagnosis when clinical and imaging analyses are inconclusive. For patients with diagnosed MS, laboratory testing is utilized to determine appropriate treatment, monitor medication side effects, and monitor for conditions such as leukopenia and/or lymphopenia.
Quick Answers for Clinicians
Autoantibody testing is important to differentiate between multiple sclerosis (MS) and other similar diseases. For example, aquaporin-4 (AQP4) immunoglobulin G (IgG) serum autoantibody and myelin oligodendrocyte glycoprotein IgG (MOG IgG) autoantibody are specific for neuromyelitis optica spectrum disorders (NMOSDs) and MOG-associated disease (MOGAD), respectively. For AQP4 testing, cell-based detection methods are strongly recommended because of their greater sensitivity and specificity when compared with enzyme-linked immunosorbent assays (ELISAs). Testing for these autoantibodies is recommended in patients who present with acute central nervous system (CNS) demyelination and clinical, imaging, or laboratory features that are atypical for MS. Research to identify MS-specific autoantibodies is ongoing.
Multiple sclerosis (MS) can be diagnosed based on clinical criteria, and imaging and laboratory findings may support that diagnosis. However, it is important to consider whether other autoimmune, inflammatory, infectious, toxic, metabolic, malignant, or genetic causes may account for the patient’s symptoms. If the clinical presentation is atypical for MS, additional laboratory testing may be helpful to exclude mimics. Depending on the clinical syndrome, such testing may include aquaporin-4 (AQP4) immunoglobulin G (IgG) serum autoantibodies, myelin oligodendrocyte glycoprotein IgG (MOG IgG) autoantibodies, antinuclear antibodies (ANAs), extractable nuclear antigen (ENA) antibodies, and antineutrophil cytoplasmic antibodies (ANCAs), tests for vitamin B12, copper, ceruloplasmin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), angiotensin-converting enzyme (ACE), tuberculosis, human immunodeficiency virus (HIV), and human T-cell lymphotropic virus type 1 (HTLV-1), Bartonella serology, syphilis serology, Lyme disease serology, cerebrospinal fluid (CSF) cytology and flow cytometry, as well as additional infectious and genetic testing in the appropriate clinical context.
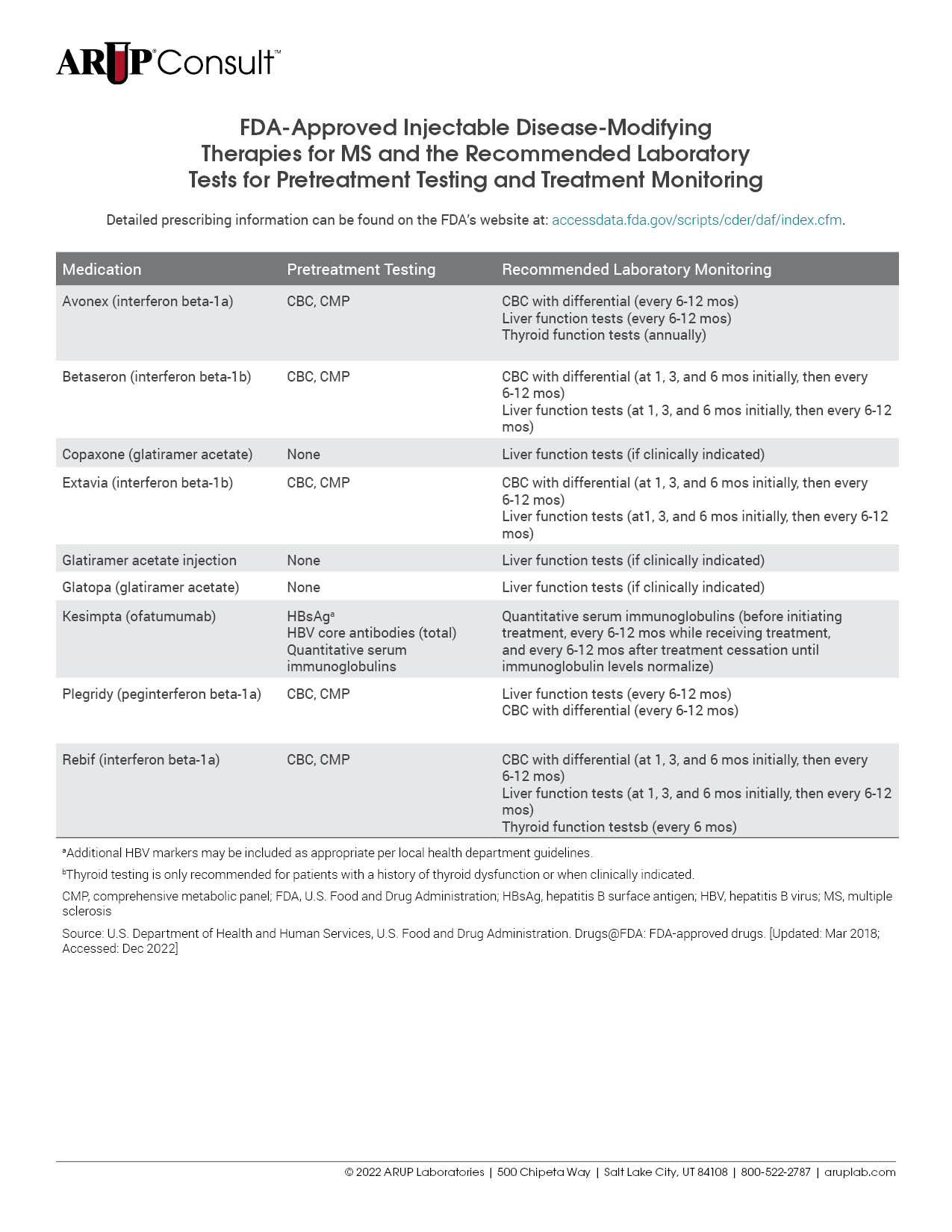
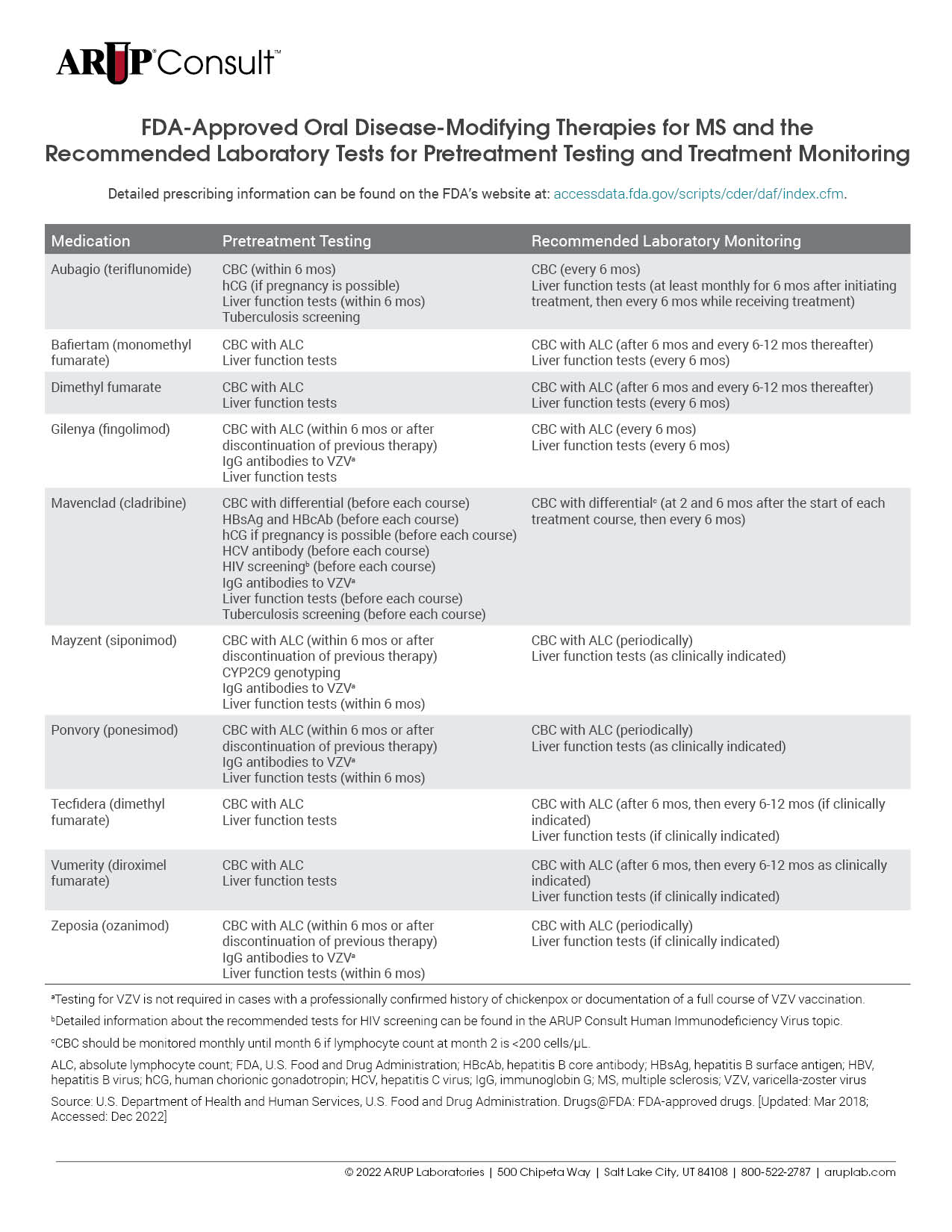
There are many treatments available for patients with multiple sclerosis (MS), and each is associated with unique laboratory testing recommendations. Testing is often recommended before treatment initiation and is used for monitoring throughout the course of treatment. Refer to our tables listing the U.S. Food and Drug Administration (FDA)-approved injectable, oral, and infusion disease-modifying therapies (DMTs) for MS and the recommended laboratory tests for pretreatment testing and treatment monitoring.
Indications for Testing
MS diagnosis is based primarily on clinical features and imaging studies. However, laboratory testing can provide supportive diagnostic evidence, particularly when clinical and MRI findings are insufficient to support a definitive diagnosis. Although laboratory testing is not required in all cases, it should be considered in the following situations :
- Disease presentation is not consistent with a typical clinically isolated syndrome (e.g., primary progressive MS)
- Clinical, imaging, or initial laboratory test results indicate features of atypical MS (e.g., clinical attacks not disseminated in space [DIS] or disseminated in time [DIT])
- Populations in which MS is less likely (e.g., children or elderly individuals)
Laboratory testing is also used to inform appropriate medical management and assess treatment response.
Diagnostic Criteria
The 2017 McDonald criteria provide guidance for the diagnosis of MS in patients with a typical, clinically isolated syndrome suggestive of relapsing-remitting MS. The criteria take into account the number of clinical attacks, when the attacks occur, the number of lesions, and the location of the lesion in the CNS. Lesions that demonstrate DIS and DIT are important for MS diagnosis when using these criteria. The McDonald criteria are also useful for patients with insidious neurologic progression that is suggestive of primary progressive MS. These criteria cannot be used to differentiate MS from other conditions. MS should not be diagnosed unless other clinically similar diagnoses have been ruled out.
Laboratory Testing
No single laboratory test can confirm the diagnosis of MS. However, laboratory testing can provide evidence in support of clinical features and imaging studies in the diagnosis of MS, particularly in cases with an atypical presentation.
Tests to Inform Diagnosis
Cerebrospinal Fluid Analysis
Oligoclonal Bands
Oligoclonal bands (OCBs) are observed when clonal populations of antibodies are present in a patient sample. The presence of CSF OCBs is highly suggestive of MS in patients with supportive clinical and imaging factors. However, OCBs may be observed in patients with NMOSDs or other neurologic diseases, autoimmune diseases, infections, or trauma. Demonstration of DIT typically requires observation of multiple clinical attacks or imaging results consistent with both active and previous demyelination. However, according to the McDonald criteria, the observation of CSF OCBs may be used to demonstrate DIT if other requirements cannot be met, thereby facilitating earlier diagnosis and treatment. In these cases, evaluating for CSF OCBs can enable more prompt diagnosis and treatment.
To determine if CSF is positive for OCBs, a matched serum and CSF sample must be assessed to detect the presence of unmatched bands on a gel. The immunoglobulin G (IgG) index may also be assessed to identify intrathecal synthesis of IgG.
Additional CSF Studies
Additional CSF studies are helpful to distinguish MS from similar diseases. CSF cell count, protein, and glucose are often normal in patients with MS, or there may be a mild pleocytosis. Abnormalities such as high leukocyte count or elevated protein levels may be suggestive of another disease process. Detailed information about the use of CSF analysis to distinguish between MS and NMSODs can be found in the ARUP Consult Neuromyelitis Optica Spectrum Disorders topic.
Autoantibody Testing
Autoantibody testing is important to rule out diseases that present similarly to MS. Aquaporin-4 IgG (AQP4) serum autoantibody is specific for NMSODs, and MOG IgG autoantibody is indicative of MOGAD. Testing for these antibodies using a cell-based assay is generally recommended for patients with symptoms indicative of acute CNS demyelination and features that are atypical of MS to prevent misdiagnosis and improper treatment.
Pretreatment Testing
Before initiating treatment with disease-modifying therapies (DMTs), laboratory testing should be performed to minimize treatment-associated risks. The specific testing required is dependent on the DMT in question but may include a CBC with differential, liver function testing, and viral serologies. For a detailed list of MS medications and the tests recommended before treatment initiation, refer to our tables listing the U.S. Food and Drug Administration (FDA)-approved injectable, oral, and infusion DMTs for MS and the recommended laboratory tests for pretreatment testing and treatment monitoring.
Monitoring
Therapeutic drug monitoring can be performed during treatment to monitor the efficacy and safety of DMTs prescribed for the treatment of MS. Antibodies against some DMTs such as natalizumab can develop over the course of treatment and result in serious allergic reactions or loss of treatment efficacy. Laboratory testing can identify antibodies against some DMTs and aid in medical management decisions.
Leukopenia and/or lymphopenia are common side effects of many MS treatments. The impact on lymphocyte count is specific to each DMT, and each medication has a unique monitoring plan.
Each MS treatment is associated with specific recommendations for the laboratory tests that should be performed during treatment. Refer to our tables listing the FDA-approved injectable, oral, and infusion DMTs for MS and the recommended laboratory tests for pretreatment testing and treatment monitoring.
ARUP Laboratory Tests
Qualitative Isoelectric Focusing/Electrophoresis/Quantitative Immunoturbidimetry
Semi-Quantitative Cell-Based Indirect Fluorescent Antibody
Semi-Quantitative Cell-Based Indirect Fluorescent Antibody
Semi-Quantitative Cell-Based Indirect Fluorescent Antibody
Quantitative Immunoturbidimetry
References
-
29320652
Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180.
-
29275977
Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
-
34831400
Fischer S, Proschmann U, Akgün K, et al. Lymphocyte counts and multiple sclerosis therapeutics: between mechanisms of action and treatment-limiting side effects. Cells. 2021;10(11):3177.
-
31162307
Nourbakhsh B, Mowry EM. Multiple sclerosis risk factors and pathogenesis. Continuum (Minneap Minn). 2019;25(3):596-610.
-
32030196
Simonsen CS, Flemmen HØ, Lauritzen T, et al. The diagnostic value of IgG index versus oligoclonal bands in cerebrospinal fluid of patients with multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6(1):2055217319901291.
-
31162308
Solomon AJ. Diagnosis, differential diagnosis, and misdiagnosis of multiple sclerosis. Continuum (Minneap Minn). 2019;25(3):611-635.
-
32654681
Freedman MS, Devonshire V, Duquette P, et al. Treatment optimization in multiple sclerosis: Canadian MS Working Group recommendations. Can J Neurol Sci. 2020;47(4):437-455.


 Cite this page
Cite this page Feedback
Feedback
Refer to the following ARUP Consult topics for more information that may be helpful to determine differential diagnosis: Neuromyelitis Optica Spectrum Disorders, Systemic Lupus Erythematosus, Mycobacterium tuberculosis, Tickborne Diseases, Vitamin Deficiency and Toxicity, Bartonella Infection, and Treponema pallidum.
For a detailed list of tests that might be helpful to inform or monitor treatment, refer to our tables listing the FDA-approved injectable, oral, and infusion DMTs for MS and the recommended laboratory tests for pretreatment testing and treatment monitoring.